According to Hong Kong's "Eastnet" reported on August 9, the Nanotechnology Center of Tel Aviv University in Israel recently invented an electronic tattoo that combines skin electrodes with temporary tattoo maps. Professor Haning, the director of the center, first introduced the invention at an international nanomedicine seminar in Tel Aviv. It is expected to function in areas such as public opinion gathering and medical treatment . According to reports, the new skin electrode consists of a carbon electrode, an adhesive surface and a nano-conductive polymer coating. Just attach it to the face of the monitoring object and directly read the other person's product or the signal from the facial muscle. The response of the situation is higher than the answer questionnaire. Because it is comfortable and convenient to use, it is beneficial for long-term data monitoring. In medicine, electronic tattoos can replace traditional skin electrode patches for muscle function monitoring. In the future, electronic tattoos can be used to assist people with physical disabilities to control electronic prostheses with muscle signals, which has only a slight effect on the appearance. Implanted surgical instruments pack
Medical blister is divided into three categories according to the safety of its use: the first category refers to medical devices that are safe and effective enough to be guaranteed through routine management. The second category refers to the medical devices that should be controlled for their safety and effectiveness. The third category is implants to sustain life; Potentially dangerous to the human body, its safety, effectiveness must be strictly controlled medical devices.
Different from ordinary medical device Packaging and production environment requirements
1. Production environment requirements: clean workshop of the same grade;
2. Mold requirements: aluminum alloy mold with high precision is selected for production, copper mold and gypsum mold will produce mold, only aluminum alloy mold is relatively safe mold;
3. Choose environmentally friendly sheet: ensure that the blister packaging will not affect the device after contacting it. General medical device manufacturers will carry out another cleaning and mold process when packaging;
Implanted surgical instruments pack,surgical instruments pack,Aseptic device packaging,Disposable packaging apparatus blister taicang hexiang packaging material co.,ltd , https://www.medpackhexiang.com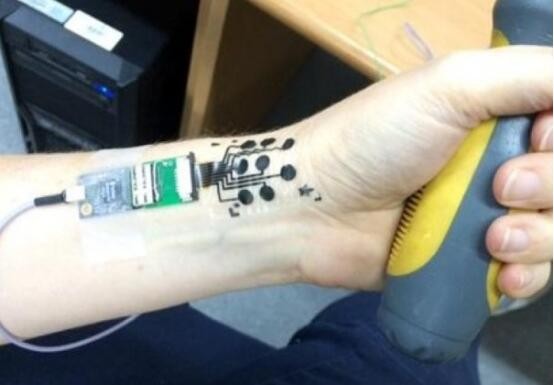

The latest development of medical electronic tattoos at the University of Israel accurately detects human emotions