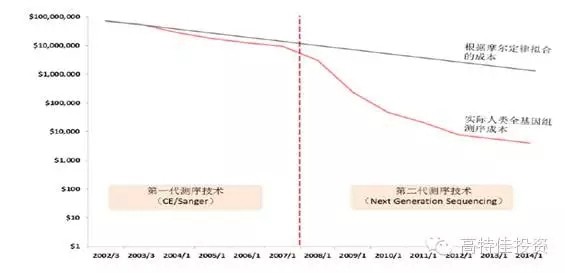
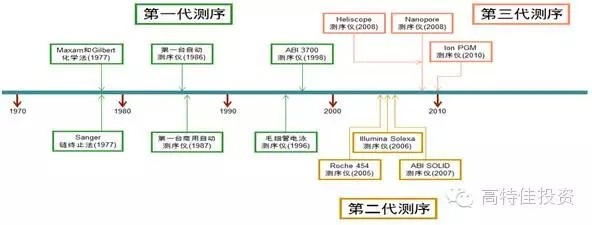
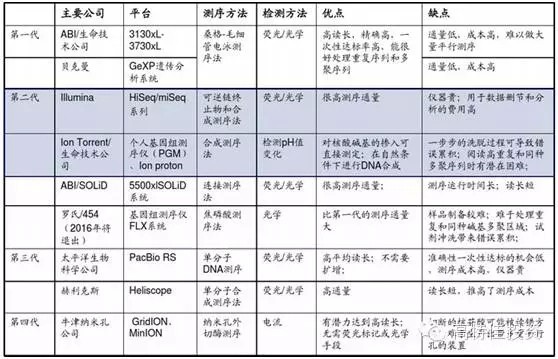
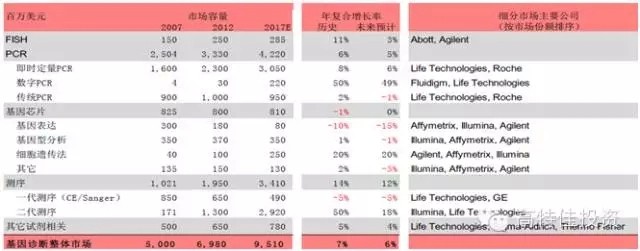
2015 will become the first year of precision medical care in China. As the front end of precision medical treatment , gene sequencing faces explosive growth opportunities, and it also faces certain challenges in practical operation. Gene sequencing is the cause of concern The development of sequencing technology and big data analysis capabilities has made precision medicine possible. Precision Medicine is based on the patient's personal genomic information to design the best treatment for patients. Compared with traditional evidence-based medicine, precision medicine is expected to become a customized medical model with maximum therapeutic effect and minimal side effects. The price of gene sequencing is reduced by the law of super-molar, making it possible to promote the economics of gene sequencing. The cost of human genome sequencing has now fallen below $1,000, and this number will continue to decline in the future; In 2008, the emergence and promotion of the second-generation sequencing technology, NGS, led to an accelerated decline in sequencing costs and significantly exceeded Moore's Law predictions. Gene sequencing technology costs drop rapidly (per megabase) The emergence and advancement of big data analysis tools enable gene sequencing to enter real-world applications For a large number of genomic data, the enhancement of big data processing capacity also provides support for analyzing and interpreting genetic data. The continuous improvement of sequencing technology and big data analysis capabilities will push precision medicine into a fast-growing track. Traditional medicine needs a breakthrough, and the policy of precision medical care boosts the development of gene sequencing Policy hurdles: In February 2015, General Secretary Xi Jinping instructed the Ministry of Science and Technology and the National Health and Family Planning Commission to request the State to establish the China Precision Medicine Strategy Expert Group. On March 11, 2015, the Ministry of Science and Technology held the first national expert meeting on precision medicine strategy and decided to invest 60 billion yuan in precision medicine by 2030. In January 2015, the state announced the pilot unit for high-throughput sequencing of prenatal screening and diagnosis. In April, the pilot unit for high-throughput gene sequencing of tumor diagnosis and treatment was announced, demonstrating the government's determination to promote the development of precision medicine. On May 14, the State Council promulgated the "Decision on Cancellation of Non-Administrative License Approval Items", and then canceled 49 non-administrative license approval items, and no longer retained the "non-administrative license approval" approval category. Relevant to medicines include: Item 31: Access approval for clinical application of the third category of medical technology; Item 52: Issuance of the administrative certificate for pharmaceuticals under the responsibility of the State Food and Drug Administration. Cancel the approval of non-administrative licenses, loosen the clinical application of the third type of medical technology, and approve the pre-existing supervision. It is expected that in the future, the approval of the implementation of the second type of medical technology implementation by the provincial health and family planning administrative department will also be phased out. Light approval, the trend of re-regulation is clear. The characteristics and development trend of gene sequencing Second-generation sequencing is still the most mainstream technology for gene sequencing At present, gene sequencing technology has been developed to the third generation (there is also the sequencing of nanoporous exonuclease into the fourth generation). The first generation of DNA sequencing technology used mainly the chain termination method pioneered by Sanger and Coulson in 1975. The second generation of DNA sequencing technology (NGS) is currently the most widely used, symbolized by Roche's 454 technology, Illumina's Solexa, Hiseq technology and Life's Solid technology. The third generation DNA technology (TGS) is represented by PacBio's SMRT and Oxford Nanopore Technologies' nanopore single molecule sequencing technology, and the sequencing process does not require PCR amplification. Second-generation sequencing is still the mainstream method of gene sequencing in 5-10 years The second-generation sequencing technology has become the mainstream of the sequencing market by replacing the first-generation sequencing technology with its high throughput, low cost and high accuracy. Because third-generation DNA sequencing technology currently faces marketization bottlenecks with high sequencing costs and relatively low accuracy of sequencing results, large-scale commercialization of TGS instruments still takes a long time. NGS has an important impact on the sequencing results: NGS sequencing process includes sample collection, gene extraction, library construction, gene sequencing and data analysis. The accuracy of each link has an important impact on the sequencing results. The size of the database directly affects the data analysis. The accuracy of the results. Second-generation sequencing technology may become the core platform for future molecular diagnosis The second-generation sequencing technology has both throughput and accuracy advantages and is expected to lead the innovation of molecular diagnostic methods. Common molecular diagnostic techniques include polymerase chain reaction (PCR), transcription-mediated amplification (TMA), fluorescence in situ hybridization (FISH), gene sequencing, and gene chip technology. Overview of market segment growth rates for major technologies in genetic testing (global) The research and development of molecular diagnostic instrument products is not based on technology but on the encouragement of innovative products. The country has opened up new channels of innovation, and the acceptance approval cycle has been greatly shortened. Molecular diagnostic techniques at different stages of development have their specific application scenarios. As the cost of NGS declines and the business model is rich, traditional molecular diagnostic methods will be challenged. The second-generation sequencing technology has the advantages of flux and accuracy, which is faster and cheaper, greatly broadens the application range of gene sequencing, and especially promotes the innovation of molecular diagnostic methods. For the organic food market, we have created a range of unique high-quality starch and sugar. All of Our ingredients are certified to applicable organic standards: National Organic Program (NOP) and European Union (EU), in order to ensure that your product benefits from optimal positioning, whether you operate on a global or local scale, we`ll work to keep you ahead of trends and improve your profitability. Plant Starch,Organic Potatoes,Organic Corn,Organic Maltodextrin Organicway (xi'an) Food Ingredients Inc. , https://www.organicwayince.com
Analysis of industrial pattern in the field of gene sequencing in 2015