PURELAB flex continuously produces ultrapure water with pure water or tap water. After installing the ELGA LabWater biofilter, ultrapure water with bioactive impurities removed can be produced. Ultrapure water with bioactive impurities removed is suitable for biochemical and molecular biology applications such as cell culture. Endotoxin interacts with cells, causing a variety of adverse effects (Reference 1 Dawson and Reference 2 Nagano). Endotoxin has a great influence on other applications such as in vitro fertilization (Reference 3 Dumoulin) and cell culture (Reference 4 Stacey). The improvement in reliability of experiments (cell division, electrophoresis, and other biochemical processes, etc.) is due to the clearance of endotoxin. At pH >2, the endotoxin is negatively charged and can be effectively removed using a positively charged filter such as the ELGA LabWater Biofilter. Charged filters have the least barrier to water flow and are best suited as end filters for pure water systems. Endotoxin challenge experiment Purified LPS is used in most endotoxin challenge experiments. ELGA LabWater's research team produces its own LPS from bacteria found in pure water. This is to mimic the realistic challenge of the experimental environment. The bacteria are first separated from pure water. The bacteria were then inoculated into peptone water and cultured at a temperature of 27 °C. The sample was repeatedly subjected to heat treatment, and filtered with a 0.45 μm filter membrane to obtain a concentrated endotoxin. Each challenge experiment lasted for 5 minutes, and the following total challenge experiment results were obtained: even if the challenge experiment exceeded 90 EU/ml, the total challenge experiment was close to 800,000 EU, and endotoxin could not be detected in the produced water after the biofilter (< 0.001 EU/ml). In fact, the amount of endotoxin in the influent water of the filter is extremely low (<0.1 EU/ml). In order to more realistically imitate the actual situation, pure water with an endotoxin content of 1 EU/ml (nominal) was used as a water inlet, and the biofilter was tested for a long time: endotoxin could not be detected after flowing 800 liters ( <0.001 EU/ml). The presence of such impurities in pure water can cause serious interference in the experiment. However, ion exchange resins and UV lamps can remove them. The uniquely designed pure water system for regular sterilization and maintenance does not contain such impurities in the produced water. This test uses the enhanced Ambion® Alert test program, which is based on the detection of cleavable fluorescently labeled RNase or DNase matrices. The test results showed that the detection content of RNase was low, reaching <0.002 ng/ml, and the content of DNase was <20 pg/ml. Even with such high impurities, PURElAB flex (with biofilter) can effectively remove RNase and DNase (RNase<0.002 ng/ml, DNase<0.02 ng/ml). Pure water produced by PURELAB flex (with biofilter) can replace the use of diethylpyrocarbonate (DePc) for water treatment (as recommended by ELGA LabWater, it has limitations on sterilization and maintenance). TOC and resistivity flushing New biofilter TOC and resistivity flush Bacterial challenge experiment to sum up references ELGA LabWater Veolia Water Treatment Technology (Shanghai) Co., Ltd. ELGA is the brand name of the global laboratory water treatment system for Veolia Water Technologies. The information in this document belongs to Veolia Water Treatment Technology (UK) Co., Ltd., which is operated under the name ELGA LabWater. ELGA LabWater and Veolia Water Treatment Technology (UK) Co., Ltd. assume no responsibility for any errors or omissions that may occur in the text. The Laser Distance Meter is precise measuring tools with high accuracy and 100m long distance. The laser distance measurer can be used indoor and outdoors. It's widely used in many areas, specially in construction industry, thanks to the IP54 dust and water resistant. The digital height measurement device Laser Distance Meter 100M,Laser Meter 100M,Laser Measure 100M Range,100M Laser Distance Meter Chengdu JRT Meter Technology Co., Ltd , https://www.rangingsensor.com PURELAB flex (with ELGA Biofilter) Performance Specifications • Endotoxin <0.001 EU/ml
• RNase <0.002 ng/ml
• DNase <20 pg/ml (<0.02 pg/μl)
• Bacteria <10 CFU/100ml (<0.1 CFU/ml) 
Endotoxin
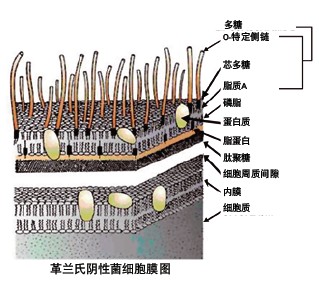
Endotoxin is a lipopolysaccharide that is detached from the outer membrane of Gram-negative live bacteria. Endotoxin is released when bacterial cells die.
The challenge to the biofilter was to continuously inject high levels of endotoxin into the positively charged filter influent, and then measure the endotoxin concentration in the produced water using the sputum deformed cell lysate test (dynamic turbidimetry). Challenge experiment (EU/ml) 0.02 2.83 14.00 48.40 90.70 Total Challenge Experiment (EU) 100 14250 84250 326250 779750 After biofilter (EU/ml) <0.001 <0.001 <0.001 <0.001 <0.001 Logarithmic reduction >1.3 >3.5 >4.1 >4.7 >5.0  DNase and RNase
DNase and RNase
Many people worry that there may be other biologically active impurities such as RNase and DNase in pure water.
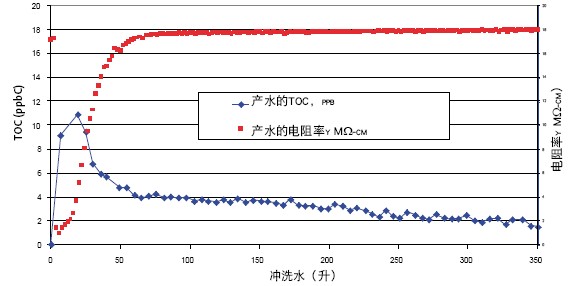
The biofilter must not contaminate water while removing any endotoxin from the water produced by the device. When the new filter is used for the first time, fast resistivity and TOC flushing are very convenient; at the same time, it is also clear that the pollution of the produced water will always be low. This is important because the purity of the water produced through the filter cannot be monitored. The biofilter's TOC and resistivity rapid rinsing are as follows:
In PURELAB flex, pure water circulates through the UV lamp and receives intense UV radiation at 254 and 185 nm to keep the bacteria content at a very low level. The remaining micro bacteria were removed using a biofilter with a bacterial filtration capacity of 0.2 μm. The biofilter completely removed the bacteria when using a solution containing 1 x 107 CFU/ml for the bacteria challenge experiment: equivalent to a log reduction factor of >8.
The figure below shows typical values ​​for the total viable count (TVC) of pure water systems (with biofilters) in 6 months. 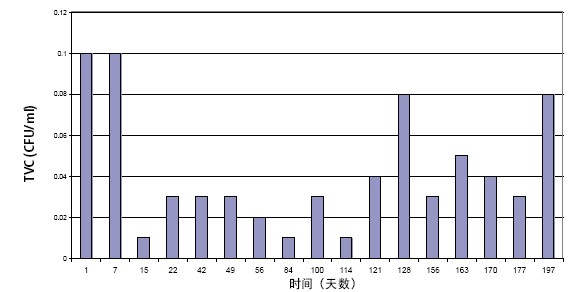
PURELAB flex (with biofilter) is extremely effective in producing ultrapure water without biologically active impurities, so it is suitable for applications such as endotoxin-free ultrapure water, bacteria-free water and nuclease-free ultrapure water.
Document 1: Dawson Me (1998) lAl update. Associates of cape cod; Vol. 16: 1-4
Document 2: Ref 2: nagano M, Takahashi y, Katagiri S (1999) J. Reprod. Dev.; 45: 239-242
Document 3: Dumoulin Jc, Menheere PP, evers Jl (1991) human Reproduction; 6: 730-734
Literature 4: Stacey g (2007) in Medicines from Animal cell culture. Stacey g, Davis J. John Whiley &Sons, chichester, chapter 31
Website:
19th Floor, Gaoteng Building, No. 318 Fuzhou Road, Shanghai 200001 China Tel: +(86)21 6391 3288
Fax: +(86)21 6391 2766
© Veolia Water Technologies (UK) Ltd., 2012 – All rights reserved. ELGA, PURELAB®, CENTRA, MEDICA®, PURESURE are trademarks of Veolia Water Technologies (UK) Ltd.
Why Our 100m Laser Distance Measurer?
1. Long range: 0.03-100m.
2. High Accuracy. The accuracy can achieve ±1.5 mm.
3. Multiple Functional Designs: can be used to measure distance, area, volume, angle, and Pythagorean measurement. Also can add function: Bluetooth, USB charge, voice, touch screen etc.
4. IP54 waterproof rating and dustproof
PURELAB flex (with biofilter) removes endotoxin, RNase, DNase and bacteria
Next Article
How to rear reserve goose
Prev Article
Winter Rabbit Breeding "Four Qiao"