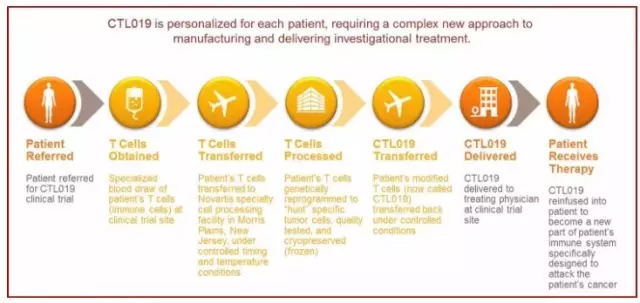
Kite Pharma and Novartis's CAR-T cell therapy targeting CD-19 antigens have entered the final stage of FDA approval, and they are expected to be the first FDA-approved CAR-T cell therapies in 2017. Recently, the two companies also released the latest clinical trial data analysis results of their respective therapies at the 22nd annual meeting of the European Hematology Association. Kite Pharma is the result of a multicenter clinical phase 2 trial using axicabtagene ciloleucel (axi-cel, KTE-C19) for the treatment of refractory, aggressive non-Hodgkin's lymphoma (NHL). Axi-cel was awarded the FDA's priority review in May this year. In a clinical trial called ZUMA-1, 101 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL) or transformed follicular lymphoma Axi-cel treatment at a dose of 2X106 cells/kg body weight was received. The tumors of these patients are all refractory or recurrent tumors. The ZUMA-1 trial achieved its primary endpoint: an objective response rate (ORR) of 82% in the modified intent-to-treat population, with a complete response rate (CR) of 54%. Important variables such as clinical tumor subtype, refractory degree, tumor grade, and international prognostic index of lymphoma did not significantly affect their clinical response. Axi-cel achieved a CR level 7 times higher than the historical control CR level. And in an average of 8.7 months of follow-up, about half of the patients still have a clinical response. Novartis Announces the results of an interim analysis of open-label, multicenter clinical phase 2 trials in patients with relapsed or refractory DLBCL using CTL019. CTL019 was awarded the FDA's priority review qualification in March this year, and in April it received the FDA-issued breakthrough therapy award. In a clinical trial called JULIET, 85 patients in 10 countries received a CTL019 treatment. The tumors of these patients still relapse or worsen after having received at least two different therapies, and they are not suitable for stem cell transplantation therapy. The JULIET clinical trial also reached its primary endpoint. Among the 51 patients who followed the trial for more than 3 months or terminated early, the best overall response rate was 59%, including 43% CR and 16% partial response (PR). All patients who achieved complete remission were still in complete remission at the time of data cut-off. â–²CTL019 treatment process (Source: Novartis official website) The frequency of the ultrasonic energy meter is 1 megahertz (1 million Hz / s), the direction is accurate, and the penetration is strong. Through the three major effects of sound waves (mechanical, warm, physical and chemical effects) on various parts of the human body, to achieve the role of massage relaxation. Ultrasound Treatment, Healthcare Ultrasound, Medical Ultrasound, Therapeutic Ultrasound Shenzhen Guangyang Zhongkang Technology Co., Ltd. , https://www.nirlighttherapy.com

Kite Pharma and Novartis Announce New Results in Clinical Trials of CAR-T Therapy
Next Article
How to raise sheep's efficiency?
Prev Article
Woman breast-feeding so eat the best