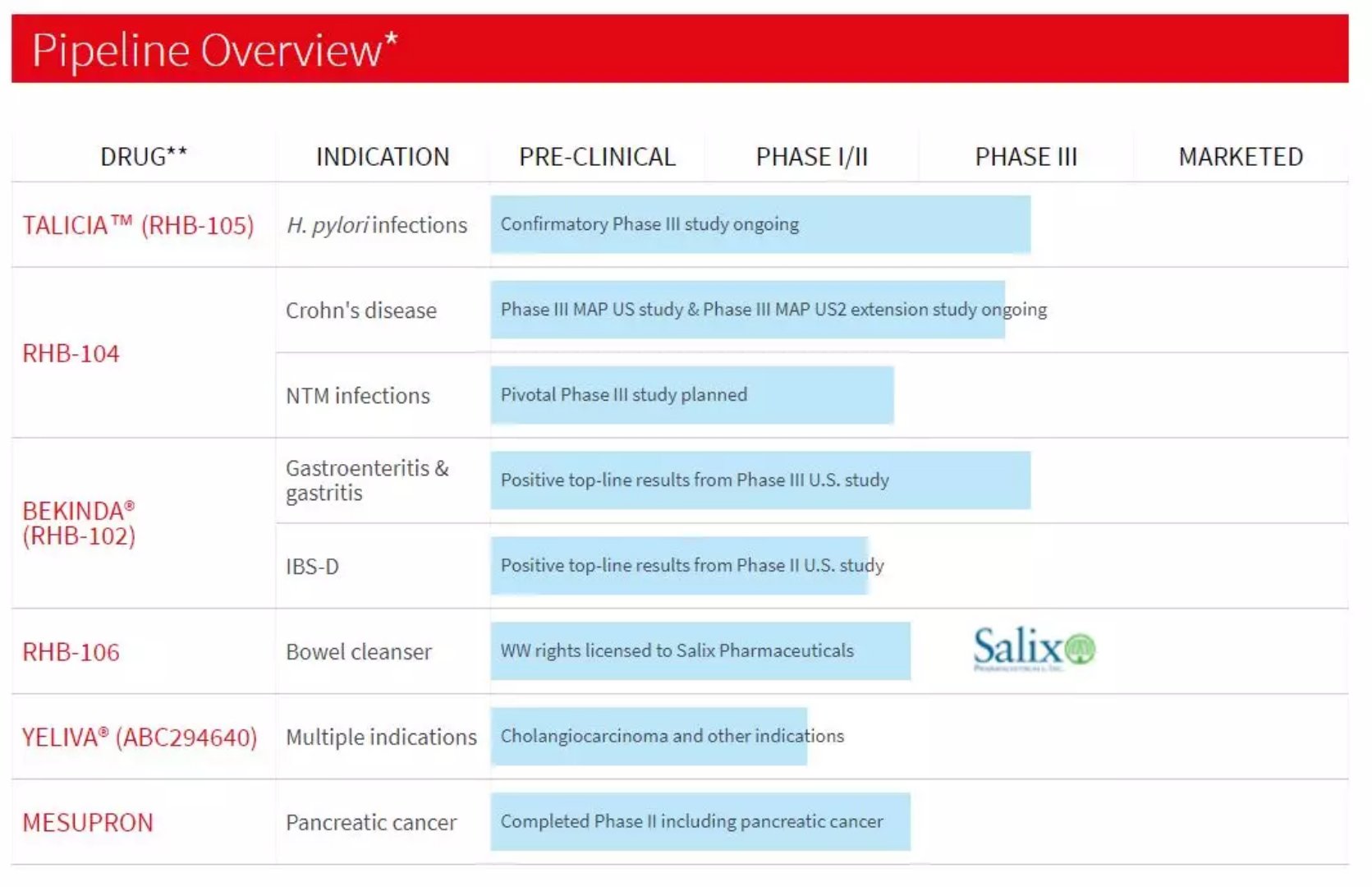
The second phase of the new drug of irritable bowel syndrome reached the primary end point January 18, 2018 Source: WuXi PharmaTech Biomedical company RedHill recently announced the final results of Phase 2 clinical trials of BEKINDA (RHB-102) for the treatment of diarrhea-type irritable bowel syndrome (IBS-D). Irritable Bowel Syndrome (IBS) is one of the most common gastrointestinal disorders affecting approximately 30 million Americans. About 40% of cases are diarrhea-type irritable bowel syndrome (IBS-D). Between 2013 and 2016, the US IBS-D treatment market grew by approximately 550%. BEKINDA® is a 24-hour, two-phase, sustained-release, ondansetron oral preparation that is protected by multiple patents. The primary endpoint was successfully achieved in a Phase 3 clinical study (GUARD study) of BEKINDA® 24 mg for the treatment of acute gastroenteritis and gastritis. A randomized, double-blind, placebo-controlled phase 2 clinical study in the United States recently evaluated the efficacy and safety of BEKINDA® 12 mg. The study enrolled 126 adult patients over the age of 18 who received BEKINDA® 12 mg or placebo once daily for eight weeks. ▲ RedHill's product pipeline overview (Source: RedHill official website) An independent review and analysis of the final results confirmed that the BEKINDA® 12 mg Phase 2 clinical study successfully reached the primary endpoint. Significant improvement in the primary efficacy endpoint - fecal traits (as defined by the FDA guidelines) - increased by 20.7% compared with placebo (p = 0.036). The final top line results were improved based on previously published top line results (absolute difference 19.4%, P = 0.05). Phase 2 studies showed that BEKINDA was superior to previously reported XIFAXAN® (rifaximin) and Viberzi® (eluxadoline) at all three efficacy endpoints. RedHill said it plans to meet with the FDA in the first half of 2018 to discuss the design of one or two BEKINDA® 12mg key Phase III clinical studies for IBS-D. We look forward to more effective new drugs to alleviate the discomfort of patients with irritable bowel syndrome. Reference materials: [1] RedHill official website [2] RedHill Biopharma Announces Final Results from Phase II Study with BEKINDA for IBS-D Alginate Dressing,Silver Alginate,Alginate Wound Dressing,Alginate Dressing For Wound Care Henan Maidingkang Medical Technology Co.,Ltd , https://www.mdkmedical.com
The second phase of the new drug of irritable bowel syndrome reached the primary end point