Stenosis Ureteral Stent,Ureteral Stent,Urethral Catheter,double j catheter Anesthesia Medical Co., Ltd. , https://www.honestymed.com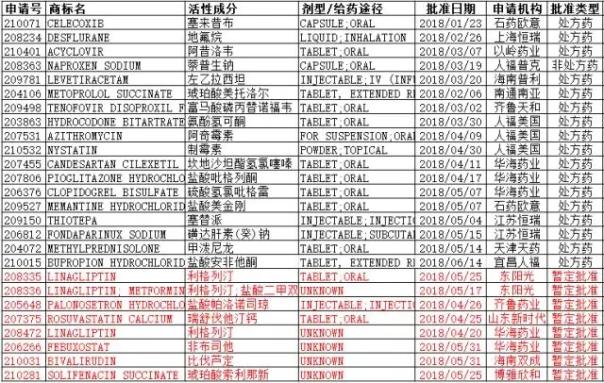
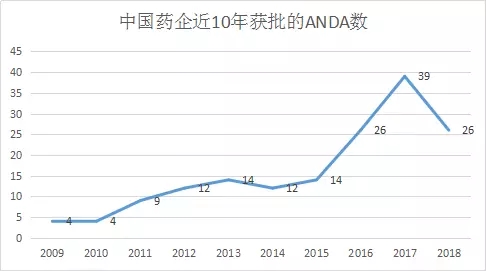
In the first half of the year, Chinese pharmaceutical companies received 26 FDA-approved ANDAs.
Medical Network June 28th In the first half of 2018, Chinese pharmaceutical companies received 26 FDA approvals for ANDA, an increase of 38.5% year-on-year (16 in the first half of 2017), a total of 25 active ingredients from 15 Chinese pharmaceutical companies. . At the same time, statistics show that the total number of ANDAs approved by Chinese companies for FDA has reached more than 250 application numbers.
   Table 1: ANDA approved by the FDA for Chinese pharmaceutical companies in the first half of 2018
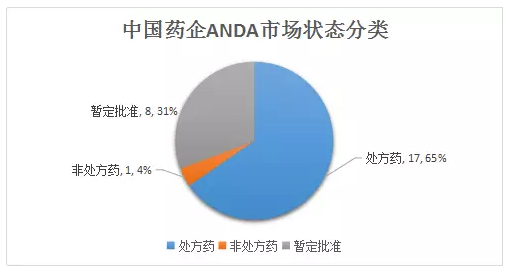
The ANDA on the icon red was tentatively approved, reaching 8 in the first half of 2018, accounting for 31%. The FDA's provisional approval means that it cannot be listed in the US due to patent and/or exclusivity reasons, but it meets the FDA's quality, safety and efficacy standards listed in the US.
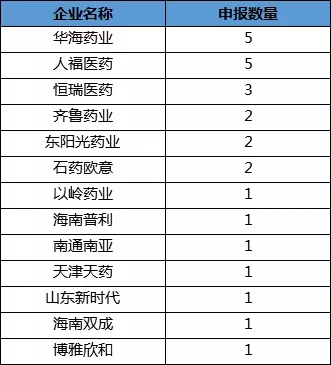
In 2018, many new faces were ushered in the internationalization of Chinese pharmaceutical companies, such as Yiling Pharmaceutical, Tianjin Tianyao, Shandong New Era, Hainan Shuangcheng, and Boya Xinhe. Up to now, 35 Chinese pharmaceutical companies have obtained the FDA-approved ANDA.
Zhejiang Huahai Pharmaceutical has achieved five FDA-approved ANDA drugs through its US subsidiary, Princestone, which is worthy of the leading company of Chinese pharmaceutical internationalization. Not long ago, Huahai Pharmaceutical’s valsartan was approved, and the variety was completed with the policy dividends such as “US listed but not listed in China, using overseas data declaration, and passing the consistency assessment according to the new 4 generic drugs approvalâ€. Over-the-counter oral solid preparations.
Renfu Medicine also received 5 ANDA numbers, but its applicants were from EPICPHARMA (3), People's Fouc (1) and Yichang Renfu (1). EPICPHARMA is a US company wholly acquired by Renfu Pharmaceutical in 2016. It will calculate the post-acquisition approval for Renfu Medicine.
Hengrui Medicine, Qilu Pharmaceutical, Dongyang Sunshine, Shiyao Ouyi and other old-fashioned international pharmaceutical companies are not willing to show weakness, respectively, three, two, two, two ANDA. The remaining seven pharmaceutical companies obtained an ANDA number in the first half of 2018.
In general, the ANDA approved in the first half of 2018 hit a record high, and the dividends of the China-US double newspaper became more and more obvious. In the second half of the year, more companies will report to the United States. I hope domestic pharmaceutical companies can get an NDA drug in the United States as soon as possible.
Finally, give everyone Amway an ANDA query method approved by Chinese pharmaceutical companies. Enter the Pharmacy US FDA drug database, find the "Chinese pharmacy" search bar in the search box, select "Yes" in the drop-down option, all the ANDA are screened out, is it very simple?
Next Article
Greenhouse Chayote Seedling Technology