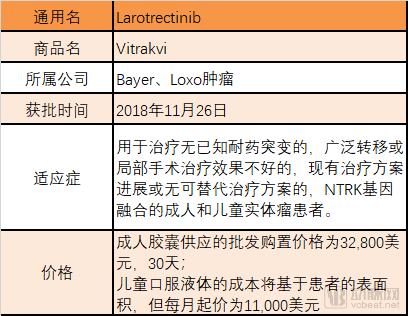
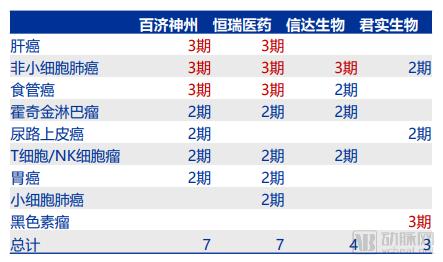
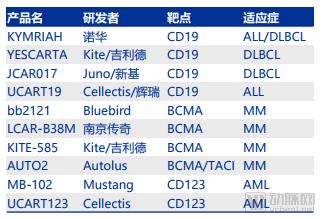
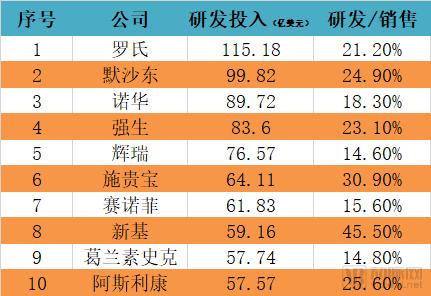
Recently, the discussion caused by the FDA-approved “broad spectrum anticancer drugs†may become a classic case of communication. First of all, cancer is a national topic and it is very easy to attract everyone's attention. Secondly, cancer is complicated and treatment is diverse. Everyone hopes to have a "clear solution" to overcome cancer. Finally, information transmission is getting faster and faster. The more direct it is, the lack of professional “gatekeepersâ€, the influence of one message can be amplified exponentially. After the news of the broad-spectrum anticancer drug came out, the arterial network contacted the relevant manufacturer Bayer and the industry experts for the first time to interpret the incident and the drug itself. The article is divided into four parts: 1. Experts questioned why "the god medicine" is not god; 2. "God medicine" real face analysis, what are the limitations; 3. Broad-spectrum anti-cancer drugs are starting to take off, and what kind of research and development and product reserves are being carried out by major pharmaceutical companies at home and abroad; 4. Fighting cancer is still on the road. Experts answer, "God medicine" is not god Arterial Network interviewed Mr. He Xiaobing, President and CEO of Mingyan Medicine, and gave an explanation of the broad-spectrum anticancer drug incident. Arterial Net: Does a broad-spectrum anti-cancer drug really have a "magic" effect, can "cure" so many cancers, and some reviews say that "response" is high. In terms of actual effects, what is the effect of this drug? A: The first thing that should be clear is that cancer cannot be “cured†and the current treatment goal is to prolong the overall survival of patients. The "response" can be understood as that the tumor cells respond to the drug being used. The so-called broad-spectrum anticancer drugs actually mean that they have effects on many different types of tumor targets. Among these targets, some targets are more sensitive to the drug, and the therapeutic effect may be more significant, such as a decrease in tumor markers and a tumor shrinkage. Some targets are not so sensitive to the drug, and the effect is not so significant. It may be manifested as tumor control does not grow up, and the disease does not deteriorate. These are clinically positive, as they extend the patient's survival time. On the other hand, when a patient performs genetic testing with a tumor tissue, the result shows how much the mutation rate of a certain target is. If the target with a high mutation rate is just a target that is sensitive to the drug, a good therapeutic effect will be obtained. If the target with a high mutation rate is not within the target range of the drug, the effect is not satisfactory. Patients should not be superstitious about broad-spectrum anticancer drugs during the course of cancer treatment. You should look for the most sensitive drugs for treatment in light of your own situation. Arterial Net: In the horizontal direction, in addition to Bayer, there are no other companies that have a layout on “broad spectrum†anticancer drugs, including those that have been approved and are being developed (clinical). A: From the perspective of scientific research, the term “broad spectrum anticancer drugs†is not very strict. If a drug that applies multiple cancers is called a broad-spectrum anticancer drug, it is currently laid out on a broad-spectrum anti-cancer drug. There are still a lot of companies, such as the "broad-spectrum" anticancer drugs that are currently on the market and have a large number of clinical data proven to be effective, which are PD-1 drugs, including MSB's Pabolizumab and BMS's Nawubizumab. They actually target only one target, but this target is on the body's immune system, so it can arouse the body's own resistance to tumor tissue, thereby playing a role in eliminating cancer cells. In addition to MSD and BMS, Roche has Atezolizumab. In addition, Pfizer and AstraZeneca have layouts in the PD-1 field, but they are not listed. The PD-1 mentioned earlier belongs to biological macromolecules. In the field of small molecules of chemical drugs, there are also "broad spectrum" anticancer drugs, which are aimed at multiple targets. For example, cabotinib, which was listed some time ago, Larotrectinib, which was just approved for marketing by the FDA, and erlotinib developed by China's Zhengda Tianqing. This type of "broad spectrum" anticancer drug may appear in my previous situation, with different degrees of sensitivity to different targets, which may lead to the fact that for some cancers, it may be a first-line drug. For some cancers, it may only be a second- or third-line drug. Arterial network: Whether the "broad spectrum" anticancer drug is a great advancement in medicine and pharmacy. In the vertical direction, there are milestones and landmark events in pharmacy (nearly 20 years). A: The broad-spectrum anti-cancer drug itself is a targeted drug, but it targets many targets, or it can act on a variety of cancers for a single target. The emergence of targeted drugs is a major milestone in the medical and pharmaceutical world, and they make cancer treatment more precise. Novartis's treatment of leukemia, Gleevec, which is the drug in the movie "I am not a drug god", is the world's first tumor-targeted drug. Its research and development process is very tortuous, and it took several decades to get approved. The drug brought five world-class scientists to the United States. Later, the development of biopharmaceuticals was also a major milestone, as the scope of drug development was broadened to another area. And for the development of biopharmaceuticals, it is also an area where major pharmaceutical companies compete for competition. Because there may be many difficulties in the small molecules of chemical drugs, there are opportunities to find answers in biological macromolecules. For example, Roche's Herceptin, a targeted drug for breast cancer, has actually increased the lifespan of breast cancer patients because of its remarkable efficacy. As the targets that cause tumors continue to be discovered, pharmaceutical development scientists are also increasing their efforts to develop new targets. It is believed that more tumors will be available in the future. Analysis of the true appearance of "Shen medicine" The US FDA announced the approval of larotrectinib on November 26, the original title is "FDA approved Larotrectinib for the treatment of NTRK gene fusion solid tumors." Image source: FDA official website Larotrectinib is used to treat adult and children with solid tumors with NTRK gene fusion in the treatment of patients with no known drug-resistant mutations, extensive metastasis or local surgery, and existing treatment regimens or alternative treatment options. According to Bayer's data, NTRK gene fusion is a chromosomal alteration, which results in a conformationally activated abnormal TRK fusion protein, which acts as a tumor-driven factor and promotes tumor cell proliferation and survival in tumor cell lines. Developed by Bayer and Loxo Oncology, Larotrectinib is a TRK inhibitor with central nervous system activity that specifically inhibits these proteins. TRK fusions have been found in many types of tumors in adults and children. In support of this approved clinical trial, Larotrectinib has shown clinical benefits in a variety of unique tumor types, including lung cancer, thyroid cancer, melanoma, GIST, colon cancer, soft tissue sarcoma, parotid tumor, and infant fibrosarcoma. . The diagnosis of TRK fusion tumors can be identified by specific detection methods, including the use of next-generation sequencing technology (NGS) and fluorescence in situ hybridization (FISH). Patients with NTRK gene fusion in tumors are eligible for treatment with Larotrectinib. “Larotrectinib's approval for TRK fusion cancer patients brings the first treatment, which is the result of many years of hard work and research. TRK fusion is rare, but it exists in many different tumor types. In the age of medicine, we are fulfilling Bayer's commitment to provide value to patients and doctors and to advance the future of cancer care.†Robert LaCaze, head of the Oncology Drugs Division Executive Committee and head of the Oncology Strategy Business Unit, said, “For those with NTRK gene fusion It is very meaningful to provide a specialized treatment plan for patients with advanced solid tumors." "NTRK gene fusion is a rare cancer driver, and FDA approval of Larotrectinib is an important milestone in the treatment of such tumors. I have personally witnessed the treatment of Larotrectinib, which is specifically tailored for this carcinogenic driver gene. And regardless of the patient's age and tumor type," said Dr. David-Hyman, director of early drug development services at Sloan-kettering Cancer Center and global research director of a clinical trial at Larotrectinib. "Now, we have the first Batch of treatments for this genetic alteration that are not associated with tumor type." Larotrectinib's main focus is on its scope of application, cure rate and price. First of all, Larotrectinib does apply to a variety of solid tumors, but as a targeted drug, it must detect a specific gene fusion - that is, NTRK gene fusion will be effective, according to Loxo CEO Josh-Bilenker in the Washington Post In the interview, NTRK mutations occur in less than 1% of most solid tumor types, but are common in malignant tumors such as adult salivary carcinoma and infant fibrosarcoma. In the United States, only 2,000 to 3,000 people are estimated to have NTRK-related cancer each year. Secondly, regarding the "cure rate", the correct statement should be the remission rate. The experimental data obtained by Larotrectinib showed that it carried out a clinical trial with a sample size of 55 people, and the overall remission rate in many types of solid tumors in adults and children. (ORR) was 75% (95% CI, 61%, 85%) [22% complete response (CR) and 53% partial response (PR)]. Finally, its pricing, the official pricing is 30 days of adult capsule supply wholesale purchase price of 32,800 US dollars, the cost of children's oral fluid will be based on the patient's surface area, but the starting price of 11,000 US dollars per month. However, it is currently expected that most insurance companies will set the drug and patient's out-of-pocket expenses at $20 or less per month. Bayer said it will provide payment assistance to patients in need, and if the drug does not provide clinical benefit in the first three months, they will refund the drug to the payer and the patient. List of main information of Larotrectinib Source of data: Bayer's official website, public information In addition, like many drugs, long-term use of Larotrectinib also produces resistance, and Bayer and Loxo are currently developing second-generation drugs for new mutations. "Broad-spectrum anti-cancer drugs" As mentioned earlier, as long as a drug is suitable for a variety of diseases, it can be named "broad spectrum." For example, the broad-spectrum anticancer drug carmustine, which has been used in clinical practice for more than 40 years, has a high molecular weight, a strong lipophilicity of β-chloroethyl, and a low plasma protein binding rate, which can quickly pass the blood-brain barrier. It is mainly used for the treatment of brain tumors, metastatic brain tumors and other central nervous system tumors. It is the primary drug for primary brain tumors (such as glioblastoma) and secondary tumors. In recent years, carmustine has also developed a combination of drugs to treat gastric cancer, rectal cancer, bronchial cancer, etc., clinical development is prosperous. As well as the famous paclitaxel, paclitaxel is a broad-spectrum cycle-specific anti-tumor drug with a unique mechanism of action, which promotes the assembly of microtubule dimers into microtubules, while stabilizing microtubules by preventing depolymerization, preventing cells Mitosis inhibits cell proliferation, promotes hypoxia-oxygenation and anti-tumor angiogenesis, and enhances tumor cell response to radiation. Indications include first-line and second-line treatment of ovarian and breast cancer and non-small cell lung cancer (NSCLC). And treatment of head and neck cancer, esophageal cancer, seminoma, recurrence of non-Hojin's lymphoma, etc. Coccimycin in antibody drug conjugates (ADC) is also an antibiotic cytotoxin that has both broad-spectrum antibacterial activity and potent killing of various tumor cells. It binds to the small groove of DNA double helix and passes through the Bergman ring. The reaction produces a benzene ring double radical, cleaves the DNA double helix backbone and kills the tumor cells. The drug developed based on calicheamicin has been used in acute myeloid leukemia, relapsed or refractory B cell precursor acute lymphoblastic leukemia. Of course, the industry's most promising "broad-spectrum anti-cancer drugs" should also be PD-1/L1 mAb, because of its broad-spectrum nature and the potential for combination with many drugs, making the market for PD-1 mAb. The future is very much looking forward to. The PD-1 monoclonal antibody currently available has O medicine, K medicine, and Libtayo (cemiplimab-rwlc), which has just been released, and PD-L1 monoclonal antibody, which has been marketed, including Rocent's Tecentriq (atezolizumab). Africone's Imfinzi (durvalumab), Pfizer / German Merck's Bavencio (avelumab). According to the Shenwan Hongyuan Research Report, the PD-1 products of Baekje Shenzhou, Hengrui Medicine, Cinda Bio and Junshi Bio have been declared in the clinical stage of multiple indications. 4 domestic PD-1 clinical progress Source: insight, Shen Wan Hongyuan Research In addition, CAR-T therapy can also be called "broad spectrum" anti-cancer technology to some extent. The therapy first extracts T cells from patients, and then uses the CAR structure to equip T cells with "precision-guided weapons" (ie, identification). The target of cancer) is implanted in a large number of patients and then implanted into the patient to obtain immunity against specific cancers. There are currently two CAR-Ts available, namely Novartis KYMRIAH (indications for acute lymphoblastic leukemia ALL and terminal DLBCL in the 25-year-old patient) and Kite's YESCARTA (indications for the terminal line of diffuse large B-cell lymphocytes) Tumor DLBCL). In addition, there are many CAR-T therapies for different targets in clinical trials. The more famous ones are Juno/New JCAR017, Cellectis/Pfizer's UCART19, Bluebird's bb2121, and Kingsray's Nanjing Legend. LCAR-B38M and so on. Part of CRT-T product progress Source: Yaodu, FDA, Shen Wan Hongyuan Research However, no matter what kind of "broad-spectrum anti-cancer drugs", there are limitations in indications, targeted drugs are not "broad spectrum", "broad spectrum" is not accurate. Fighting cancer, still on the road From the global data, the investment in R&D of highly competitive multinational pharmaceutical companies is relatively high. According to the data, the top 10 research and sales revenues of pharmaceutical companies in the world are basically above 20%, and the highest is 45%. 2017 global R&D investment TOP10 pharmaceutical companies Data source: company announcement, intranet, arterial network The global competitiveness of multinational pharmaceutical companies is not unrelated to their high R&D investment. Continuous R&D investment has spawned high-quality drug production and created a “blockbusterâ€. A drug from some pharmaceutical companies can achieve sales revenue of over 10 billion US dollars worldwide, and lead the market for several years, bringing huge profits to the enterprise. Whether it is the "I am not a drug god" that was popular among the people before, or the "broad-spectrum anticancer drug" that has recently been discussed, it is actually the hope of the general public to overcome disease and overcome cancer. Fighting cancer is still on the road. Hand massager Hand massager Shenzhen Jie Zhong Lian Investment Co., Ltd. , https://www.szmeizons.com
"Broad-spectrum anti-cancer drugs" big bottom: not only Bayer, how are the major pharmaceutical companies in the layout?
Next Article
How to manage citrus in winter
Prev Article
Mustard cultivation techniques