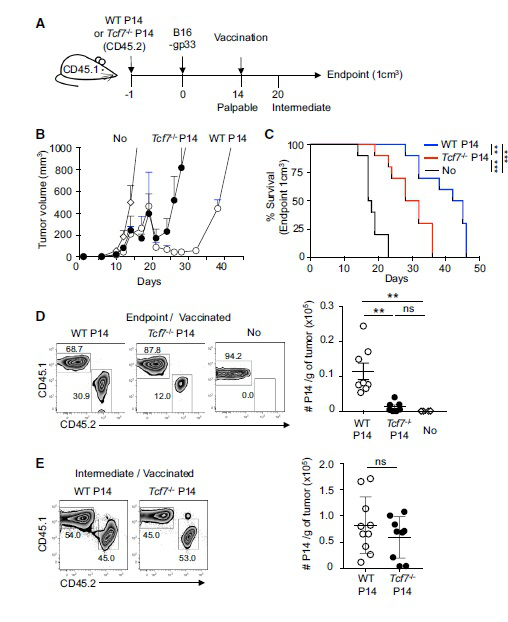
In a long-term confrontation with a virus or cancer, exhausted T cells gradually lose their function, a state known as T cell depletion. In today's hot immunotherapy, checkpoint blockade promotes tumor-infiltrating CD8 + T lymphocyte proliferation. However, the mechanism behind this phenomenon remains unclear. The research team led by Werner Held of the University of Lausanne in Switzerland has recently identified Tcf1 + PD-1 + CD8 + T cells in tumors. These cells exhibit stem cell-like characteristics and block immunotherapy in response to therapeutic vaccination and immune checkpoints to promote tumors. control. The article entitled "Intratumoral Tcf1 + PD-1 + CD8 + T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy" was published in the January issue of Immunity. Checkpoint blockade mediates the proliferative response of tumor-infiltrating CD8 + T lymphocytes (TIL). The source of this response remains unclear because chronic activation promotes terminal differentiation of T cells, that is, T cell depletion. The researchers identified a subset of tumor-reactive TILs that showed signs of depletion of cells and central memory cells, namely the expression of the checkpoint protein PD-1 and the transcription factor Tcf1. They found that Tcf1 + PD-1 + TIL mediates proliferative responses during immunotherapy, producing Tcf1 + PD-1 + cells and differentiated Tcf1 − PD-1 + cells. Ablation of Tcf1 + PD-1 + TIL limits the response to immunotherapy. Tcf1 is not required for the production of Tcf1 + PD-1 + TIL, but is essential for maintaining stem cell-like function of these cells. They detected human Tcf1 + PD-1 + cells in tumor-reactive CD8 + T cells in the blood of melanoma patients and TIL in primary melanoma. Thus, blockade of the immune checkpoint does not depend on the reversal of the T cell depletion program, but rather on the proliferation of stem cell-like TIL subpopulations. background The degree of infiltration of CD8 + T cells in certain tumors can predict the overall survival of the patient, indicating that lymphocytes can limit tumor growth. Despite this, most infiltrating tumors continue to progress, suggesting that this spontaneous anti-tumor immune response is often insufficient to control tumors. Immunotherapy aims to enhance the anti-tumor immune response to induce long-lasting tumor control. Current methods include adoptive T cell therapy and blockade of inhibitory receptors (immunization checkpoints). In long-term confrontation with viruses or tumors, the function of T cells is gradually lost (referred to as T cell depletion). They lose effector function and induce PD-1, a negative regulator of T cell function. Usually this depleted phenotype is considered to be terminally differentiated cells, which lose their ability to expand and have a short life span. However, the proliferation of CD8 + T cells in tumors after PD-1 blockade is contradictory to the concept of terminal differentiation. To this end, the researchers wanted to know whether the anti-tumor CD8 + T cell response caused by spontaneous or immunotherapy involved memory CD8 + T cell components, and if so, whether these cells are part of the tumor microenvironment. Lack of Tcf1 weakens tumor control To determine whether CD8 + T cells respond to tumors involving memory components, the researchers tested whether these responses are dependent on the transcription factor Tcf1, which is critical for the formation and function of central memory CD8+ T cells. They adoptive transfer of wild-type WT or Tcf7 - / - CD8 + T cells into naive C57BL/6 (B6) mice. One day later, B16 melanoma cells expressing LCMV gp33 were implanted subcutaneously in this P14 chimeric mouse. This method can be used to track tumor-specific CD8 + T cells and determine whether these cells and their subpopulations are involved in tumor control. When tumors are touchable, they use the gp33 peptide to immunize mice. Vaccination and a combination of WT or Tcf7 - / - P14 cells resulted in a brief regression of the tumor approximately one week later. They found that the protective effect of Tcf7 − / - P14 cells was transient compared to WT P14 cells. At the end of treatment, the abundance of tumor-infiltrating CD8 + T lymphocytes in WT was 10 times higher than that of Tcf7 - / - P14. Therefore, the P14 TIL lacking Tcf7 was highly abundant in the early stage of vaccination, but then decreased, which was associated with a decrease in tumor control. Tumor-specific TIL contains large amounts of Tcf1 + PD-1 + and Tcf1 - PD-1 + CD8 + T cells Since prolonged tumor control was dependent on Tcf1, the researchers then identified Tcf1 expression in WT P14 cells in vaccinated and untreated mice. Naive P14 cells behave as homogeneous Tcf1 + . Similarly, when untreated mice developed haptic tumors, most of the P14 TIL was Tcf1 + but also expressed PD-1. When the tumor was medium in size, the abundance of Tcf1 + PD-1 + P14 TIL increased by 8 times, but when the tumor increased to 1 cm 3 , its abundance decreased. They vaccinated the tumor-bearing mice with a therapeutic vaccine. One week later, Tcf1 + PD-1 + P14 TIL was strongly amplified (176-fold), and these cells were still present in high amounts at the endpoint. The P14 TIL also contains a significant amount of the Tcf1 - PD-1 + subpopulation, which is amplified and reduced in parallel with Tcf1 + PD-1 + TIL. They also found that Tcf1 + PD-1 + TIL is constitutively present in tumors by GFP labeling experiments. As the tumor progresses, their abundance increases instantaneously and is further amplified by therapeutic vaccination. The role of Tcf1 + CD8 + T cells in tumor control To assess the importance of tumor-specific Tcf1 + CD8 + T cells in immune response and tumor control, the researchers commissioned Cyagen to generate a mouse strain that allows selective ablation of Tcf1-expressing cells in vivo. They inserted a diphtheria toxin receptor (DTR) T2A GFP fusion gene into BAC containing the Tcf7 locus to prepare Tcf7 DTR-GFP transgenic mice. Next, they generated Tcf7 DTR-GFP P14 chimeric mice and implanted B16-gp33 tumor cells. Diphtheria toxin (DT) treatment was effective in removing GFP + P14 TIL, but had no effect on the abundance of GFP - P14 TIL. They then treated the tumor-bearing mice with DT and immunized again. DT exposure resulted in the almost disappearance of P14 cells expressing GFP or Tcf1 protein, while the abundance of Tcf1 - P14 cells was also reduced. This indicates that Tcf1 + cells maintain the production of Tcf1 - TIL. The Tcf7 DTR-GFP P14 chimeric mice had a lower tumor volume and weight when sacrificed. In contrast, tumors progress when Tcf1 + P14 cells are ablated. These data indicate that tumor-specific Tcf1 + PD-1 +  CD8 + T cells are required for the formation of anti-tumor immunity. Thus, tumor control by vaccination of therapeutic vaccines is dependent on the presence of tumor-specific Tcf1 + PD-1 + CD8 + T cells. Later, the researchers confirmed through a series of experiments that it is indeed the contribution of intratumoral cells rather than peripheral cells. Response to immune checkpoint block therapy In animal models and cancer patients, blockade of co-repressor receptors such as PD-1 and CTLA-4 can mediate T cell-dependent tumor regression. However, the molecular mechanisms behind this therapeutic intervention remain unclear. The researchers speculate that Tcf1 + CD8 + T cells may play a role in immune checkpoint blocking therapy. They used Tcf7 DTR-GFP P14 chimeric mice, used FTY720 to prevent new T cells from flowing into the tumor, and used diphtheria toxin (DT) to ablate Tcf1 + P14 TIL. Checkpoint blocking therapy increased the abundance of Tcf1 + and Tcf1 - P14 TIL by more than 10 fold compared to control mice. Elimination of Tcf1 + TIL greatly reduced the abundance of Tcf1 - P14 TIL. Thus, checkpoint blockade amplifies intratumoral Tcf1 + TIL, which are required for the production of differentiated Tcf1 - TIL. Tcf1 + PD-1 + CD8 + T cells in tumors have the characteristics of stem cells and depleted cells Given the importance of Tcf1 + PD-1 + TIL for tumor control, the researchers performed molecular identification of these cells. They found through functional analysis that Tcf7 GFP+ TIL has the potential to amplify, differentiate and regenerate Tcf7 GFP+ cells. Consistent with these stem cell-like features, RNA-seq analysis also revealed that the transcript of Tcf7 GFP+ P14 TIL overlaps significantly with some of the genes expressed by adult stem cells or hematopoietic stem cells. At the same time, Tcf7 GFP+ TIL also expressed multiple depletion markers (Lag3, Pdcd1, CTLA4), and Tcf7 GFP - TIL also selectively expressed more depletion-related genes, indicating that the expression of these genes is related to differentiation. Tcf1 + PD-1 + CD8 + T cells in melanoma patients Later, the researchers analyzed peripheral blood CD8 + T cells and primary melanoma in patients with melanoma, and indeed detected Tcf1 + PD-1 + CD8 + cells. Single-cell RNA-seq data indicated that human melanoma contains TCF7 + PDCD1 + CD8A + TIL, and its gene expression profile is similar to that identified in the mouse model. Through a meta-analysis of the TCGA database, they believe that TCF7/PDCD1 characteristics are associated with improved patient survival. The researchers believe that this study revealed that intratumoral CD8 + T cells co-expressing Tcf1 and PD-1 play a key role in immunotherapy. The findings help people understand how immune interventions affect the immune response to cancer. Identification of these stem cell-like cells is expected to further improve all types of cancer immunotherapy. Original search Intratumoral Tcf1+PD-1+CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy DOI: https://doi.org/10.1016/j.immuni.2018.12.021 Ice Cream Cone 26 Degrees,Cocoa Powder With Crispy Cones,Rich Cocoa Aroma Crisp Cone,Corn Ice Cream Crunchy Cone Tianjin Yongkang Food Co., Ltd , https://www.yongkangfood.com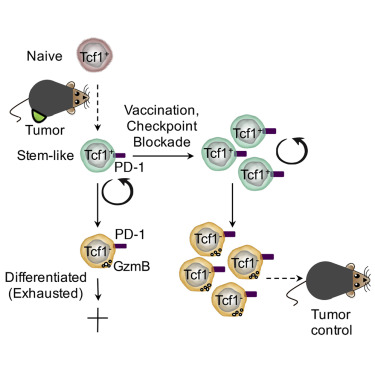
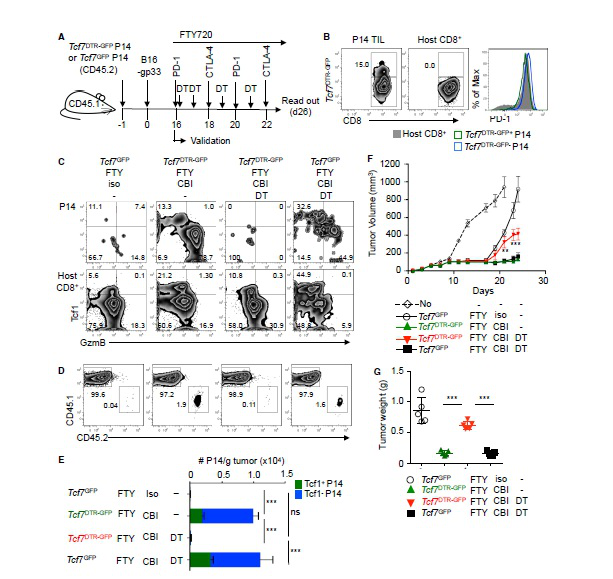
Immune checkpoint block therapy, originally relied on these cells