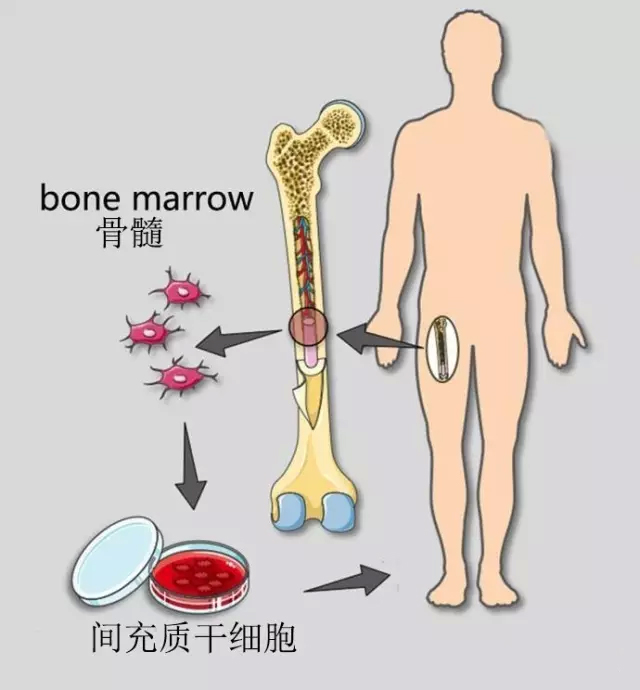
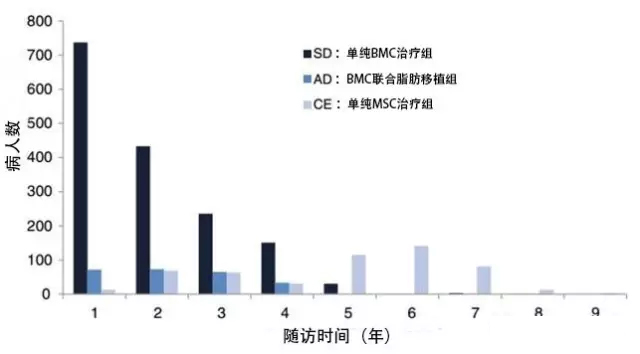
Autologous mesenchymal stem cells (MSC) have been used for the treatment of degenerative and post-traumatic orthopaedic diseases for more than 20 years. Because MSC can differentiate into bone cells, chondrocytes, muscles, tendons and ligamentous tissues, it repairs damaged tissues in time by paracrine and other unknown mechanisms of action, bringing hope and dawn to the treatment of orthopedic diseases. One of the currently popular treatment methods is to extract bone marrow, isolate bone marrow mononuclear cells (BMC), and then directly inject them with these BMC cells. The BMC cells thus obtained are not identical to mesenchymal stem cells (MSCs) but a mixed cell population including MSCs, hematopoietic stem cells, endothelial progenitor cells, macrophages and platelets. These BMC cells were purified in vitro for about 2 weeks to obtain higher purity mesenchymal stem cells (MSCs). The article summarizes and analyzes 2372 orthopedic patients undergoing stem cell therapy with a maximum follow-up of 9 years. Orthopaedic patients who received percutaneous injection of stem cells between December 2005 and September 2014 were collected from 18 clinical institutions in Australia and the United States. Injection sites include knees, hips, feet/skulls, hands/wrists, elbows, shoulders, and spine. The time points for follow-up observation and evaluation of efficacy were once every year at 1, 3, 6, 12 months and thereafter. According to different treatments, the patients were divided into three groups: BMC treatment group, BMC combined fat transplantation group, and simple MSC treatment group. The patient's condition is mainly degenerative joint disease and trauma, involving all the extremities of the human body. Inclusion in the patient population, excluding patients requiring surgery and severe deformities, severe spinal stenosis, severe rheumatoid arthritis, and systemic lupus erythematosus.
These products are used in direct response to the covid-19 pandemic.
The COVID-19 outbreak has resulted in an urgent need for medical products and solutions. In response, Sinoscience has expanded our production into coronavirus related products, including the VTM tube, nucleic acid extraction reagent and extractor and so on.
Covid-19 Medical Products,Vtm Test Kit,Vtm Transport Tube Kit,Vtm Specimen Tube Kit Jilin Sinoscience Technology Co. LTD , https://www.contoryinstruments.com
Analysis of autologous stem cells for the treatment of adverse reactions in orthopedic diseases