The potential and limitations of protein degradation therapy into the clinic March 12, 2019 Source: WuXi PharmaTech When Dr. Ian Taylor saw in the newspaper that an emerging biotech company would focus on developing targeted protein degradation agents, he immediately became interested in the company. Most small molecule drugs inhibit protein function by blocking the active site of the protein, and the company, called Arvinas, is looking for small molecules called "PROTACs" that use the protein's protein degradation mechanism to completely destroy the protein. “I said to myself: This is great, you can use it to target targets that cannot be used for medicine. †Dr. Taylor recalled that he was at the Pfizer Oncology Department. Today, as the senior vice president of biology at Arvinas, he will be responsible for the first clinical trial of this emerging treatment. In addition to the use of bifunctional targeting degradation agents, some companies are working to exploit other parts of the cellular proteasome mechanism to achieve the goal of degrading those non-drugable targets.
The flower type surgical lamp is designed at 2016, when it was been pushed into the market, it has good echo from the customers, because the flower type surgical lamp outlook is different with normal round types lamps, its outlook is just like a flower, it is very beautiful , also can add beauty in the operation room, the flower type ot lamp change the normal condition for baldness operation room style, can make doctor and patients feel much difference; for the flower type surgery lamps specification, it is almost same as round type surgical lamp, in the first year, it also has good selling;
Flower type surgical lamp also has three types, ceiling types which include single dome, double dome , with camera or without camera; wall type and mobile type flower operation lamp;
ot light,operation theatre light,operating light,operating room light,surgery light,surgicai lamp,Led ot light Shandong Lewin Medical Equipment Co., Ltd. , https://www.lewinmed.com
Arvinas will begin recruiting patients for Phase 1 clinical trials using ARV-110 to treat patients with prostate cancer. ARV-110 is a bifunctional molecule with one end of the molecule that binds to the target – the androgen receptor (AR) for this research. The other end of it can bind to E3 ubiquitin ligase. The ligase can label the target "labeled" with ubiquitin, allowing the target protein to be transported to the cell's proteasome for degradation. Later this year, Arvinas plans to launch a clinical trial using ARV-471 to treat breast cancer. It is a targeted protein degradation agent that targets the estrogen receptor (ER) using the same mechanism.
Targeted degradation agents from other companies are also entering the clinical development phase. Among them is Novartis, which targets targeted degradation agents that have not yet been disclosed, and will enter the clinical phase later this year. “ Everyone in the industry is holding their breath and waiting for the first clinical results of these trials, †said Dr. Jason Imbriglio, who is working on a targeted degradation agent at Merck (MSD): “These results may really change people’s A technical view." As reported by the WuXi PharmaTech WeChat team, protein targeted degradation technologies are redefining small molecule drugs.
The potential of this technology has led companies such as Arvinas, C4 Therapeutics and Kymera Therapeutics to focus on improving the chemical properties of targeted degradation agents. Large pharmaceutical companies are also investing heavily in this technology area. “Every major pharmaceutical company, and even every medium-sized biotechnology company, has in-house R&D or partnerships in this area,†said Dr. Nello Mainolfi, Kymera’s Chief Technology Officer.
"From 2019 to 2021 will be a period of eruption in this area, " said Dr. Andrew Phillips, CEO of C4 Therapeutics. 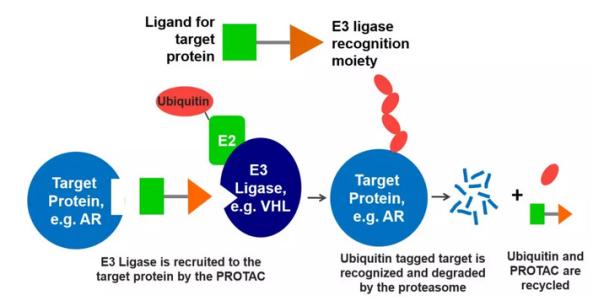
â–²PROTAC bispecific molecular mechanism of action (Source: Arvinas official website)
Scientists at Cedilla Therapeutics are exploring a broader approach. " There are mechanisms in the cell that regulate the abundance of any protein. These mechanisms also regulate dysfunction, folding errors, or the abundance of synthetic error proteins, " said Dr. Brian Jones , the company's chief scientific officer. "So our starting point is us." There is no need to manually recruit any protein, and we hope to use these intrinsic mechanisms to achieve the same effect."
In some cases, this may include finding small molecules that can degrade the stability of the protein, which initiates the protein's protein control mechanism. In addition, researchers have attempted to discover and regulate upstream factors that control protein stability and abundance, such as post-translational modification mechanisms. Moreover, disrupting the interaction between proteins and proteins and the generation of multi-target complexes may cause a target to appear "isolated" in a three-dimensional structure, thereby driving its degradation.
“ A comprehensive goal of our company is to determine the rules for protein degradation, †said Dr. Alexandra Glucksmann, CEO of Cedilla.
The main research direction of Mission Therapeutics, Forma Therapeutics and other companies is to develop inhibitors of the ubiquitin (DUB) process. Instead of recruiting E3 ligase to increase ubiquitination of specific proteins, the company's strategy is to block the removal of ubiquitin tags, thereby protecting proteins from degradation. As of September 2017, at least 15 DUB inhibitors are in preclinical development.
Design of Targeted Protein Degrading Agents <br> Targeted Degrading Agents began to enter the patent literature in 1999. At the time, researchers at Proteinix submitted patent applications using small molecule compounds to degrade specific proteins using the ubiquitin mechanism. Two years later, Dr. Craig Crews of Yale University and Dr. Raymond Deshaies of the California Institute of Technology published a peptide-based compound that induces a methionine aminopeptidase (METAP2). The occurrence of ubiquitination and degradation.
As Dr. Crews and his colleagues continue to improve their protein degradation agents, these compounds, called protein degradation targeting chimeras (PROTACs), have evolved from novel compounds to a medical model. By 2008, they could remove the components of the peptide from the molecules they developed and design a fully small molecule degrading agent. They are able to bind to AR and initiate protein degradation by letting AR come to the vicinity of the E3 ligase called MDM2. In 2013, Dr. Crews founded Arvinas to further develop this technology into the clinical phase.
In the same period, researchers began to reveal the biological mechanisms of immunomodulatory amamines, including thalidomide, lenalidomide and pomalidomide. After discovering that these compounds bind to the E3 ligase called cereblon, researchers began to consider the possibility of using them as targeted degradation agents. In 2015, Dr. Jay Bradner and his colleagues at the Dana-Farber Cancer Institute showed that they could use this activity to drive targeted degradation of BET family proteins. They named these degradants "degronimids" and co-founded C4 Therapeutics .
Regardless of the name of these degrading agents, the mechanisms of these targeted degradation strategies are like a molecular glue. They are bifunctional small molecules that bind a portion that binds to the target to a portion that recruits the E3 ligase via a link, and by binding the target protein to the E3 ligase, the specific target ubiquitin And subsequent protein degradation can occur.
Initially, however, researchers were unable to determine whether this strategy could produce small molecules that could enter clinical trials. Researchers are concerned that compounds with large molecular weights and loose structures may be difficult to optimize as compounds with high bioavailability, high specificity, and good tolerance. But as the first batch of compounds are about to enter clinical trials, their prospects are becoming brighter.
"They are molecules that look awkward and even funny," Dr. Phillips said. However, studies over the past few years have shown that " they are surprisingly well-established with normal pharmaceutical properties, " he added. Internal analysis by several companies has shown that these compounds are excellent in solubility, can penetrate into cells, can be taken orally, and are resistant to degradation by metabolic processes, and even some can cross the blood-brain barrier.
Initially researchers hoped that targeted degradation agents could become a modular platform, meaning that any target binding ligand could be paired with a "plug and play" link and E3 recruiter to form a drug. However, this desire has not yet been realized.
In contrast, industry research has shown that the ternary structure formed between the target, the degrading agent and the E3 ligase is very important. The slightest change in this structure may affect how the drug works. In some cases, this may explain why some poorly specific target binding ligands exhibit superior specificity when converted to a degrading agent. In other cases, minor changes to any component can completely eliminate the activity of the degradation agent.
“This is not a plug-and-play system,†Dr. Phillips said. “Because of synthetic strategy reasons, chemists like to split a system into every component that makes up it. But the experience of the past few hundred years has repeatedly told What we have is that a system is not a simple superposition of all the components, as is the degrading agent," Dr. Phillips said.
Therefore, C4 Therapeutics' strategy is to optimize the ability of compounds to activate the ubiquitin system, not just the ability to bring targets and ligases closer. "You certainly need to pull them together, but that's not enough. You need to activate the process," Dr. Phillips said. "We need a holistic understanding of how degradation agents work."
Dr. Mainolfi pointed out that focusing solely on the formation of ternary complexes may also be "short-sighted". In some cases, even if protein ubiquitination is successful, there is no guarantee that the target will be delivered to the proteasome for degradation. "There are a lot of things we don't know," he said.
This expression also applies to the understanding of ligases. There are approximately 600 E3 ligases in the human body, each with its own unique activity and distribution characteristics. "The recruitment of a suitable ligase to target protein is one of the keys to the success of a research and development project," Dr. Mainolfi said. If a target can be knocked out in the body without side effects, Kymera's team may choose a widely expressed E3 ligase. However, if they need a broader treatment window, the team may focus on ligases that tend to be expressed in specific tissue types or cancer cell types. The subcellular distribution of different E3 ligases is also different, which provides an opportunity to further regulate specificity.
"There are currently only five to six E3 ligases that have been publicly validated and can be used in targeted degradation agents," Dr. Mainolfi said. However, Kymera and other companies are verifying other E3 ligases in an effort to add more tools to the scientist's toolbox.
Advantages of the targeted protein degradation agent <br> degradation agent targeting the largest theoretical advantage is that they can not make the original medicine targets can be targeted drug. Small molecule inhibitors typically require blocking of the catalytic site or binding to a "pocket" that can affect protein function. However, targeted degradation agents can theoretically bind to any corner of the protein to drive protein degradation. "This opens up some of the human biology that is currently not effectively targeted by drugs," Dr. Phillips said.
However, the limitations of this approach may be overlooked. He warned: "There are still a large number of targets without any ligands that can bind to them. Without ligands, I can't build a degradation agent for you. "The long-term attractive target like Myc, even with the use of degradation technology, is still not targeted. He added.
“At the moment I think the most important question that the industry has yet to resolve is: What are the targets that can be combined with ligands?†Dr. Mainolfi said.
Dr. Bradner is optimistic about progress in this area, and he believes that most targets will eventually be targeted. “My intuition is that most protein targeting problems can be solved by small molecule discovery chemistry. This can be done by directly binding to small molecules or by cross-linking a molecule with a protein like glue. "Although this is just a feeling, I think that the targets that are currently unsolvable can be solved using this chemical." Scientists at the Novartis Center for Biomedical Research (NIBR) have successfully targeted more than 40 target-induced targets. To degradation, he said that many of these targets were once considered to be inoperable.
Due to the intense competition in this field, many companies did not disclose the targets of their research. However, we can also see the potential of this technology from several public targets.
The targets of Arvinas' two main candidates are AR and ER. Both targets are clinically validated targets, and approved therapies have been targeted at these targets. The company's CEO, Dr. John Houston, said that this is the company's founder's deliberate choice, and they believe that this has the opportunity to show that the technology of targeted degradation shows its potential and help by comparing it with other targeted drugs. A technology is optimized. Dr. Houston pointed out that although the target is not new, targeted degradation technology can still show advantages over traditional therapies.
For example, for the target of AR, approved small molecule drugs need to be combined with proteins to inhibit protein activity. Therefore, when drugs are eliminated from the body or replaced by too much protein, their activity will disappear. Most patients develop resistance to the AR antagonist enzalutamide, as cancer cells can increase levels of androgen or AR production, or produce AR mutants. These patients no longer respond to therapy.
Like other targeted degradation agents, the activity of ARV-110 is based on the catalysis of the degradation process of the target protein. This means that the candidate drug for Arvinas can be less than the target protein, and the usual small molecule inhibitors are more than the target. Reducing the dose of the drug may lead to better side effects, Dr. Talyor explained.
Another advantage of targeting degradation agents is that even if the drug is excreted, their effects can continue until the cells re-synthesize the degraded protein. "The data we have obtained from several research projects show that the catalytic activity of PROTACs allows them to degrade the new proteins that cells try to generate. This is usually one of the cancer resistance mechanisms," Dr. Taylor said. In some cases, these drugs may help delay the development of resistance.
When these clinical trials are over, attention will be paid to the absorption, distribution, metabolism, and clearance characteristics of these unconventional degrading compounds to see if their performance meets the expectations of oral administration. The field also wants to know to what extent these drugs can reduce protein levels, the rate of protein degradation and the rate of protein resynthesis, and whether these data match the data in animal models.
" The potential of targeted degradation agent molecules is that they may separate pharmacodynamics and pharmacokinetics, which means that transient degrading agents may cause long-term effects on signaling pathways, " Dr. Bradner said. "So pharmacodynamics and The relationship between pharmacokinetics will be a matter of great interest to people."
And when pioneers in this field announce the structure of drugs that are still kept secret, other researchers will quickly bring the lessons learned back to their R&D projects. “I am curious, they are optimizing which parameters to advance the preclinical project to the clinical stage. I also want to know what their compounds look like. In fact, I think their structure is degraded with ours. The structure of the agent is very different," Dr. Mainolfi said. "We hope that the clinical trial results of the first few compounds will be successful," he added.
The vast world of targeted protein degradation agents Kymera's protein-degrading agent targeting IRAK4 represents another near-term opportunity to target degrading agents. IRAK4 is a protein kinase that plays a key role in the innate immune system and is associated with a variety of cancers. Although small molecule inhibitors block the kinase action of IRAK4, this protein also has a kinase-independent effect. It can be used as a backbone protein to aid in the construction of the myddosome protein complex of the innate immune system.
"Based on genetic knockdown and knockout experiments, we believe that by protein degradation, we are able to achieve a phenotype that is completely unique and superior to inhibitors," Dr. Mainolfi said. Kymera released preclinical data on IRAK4 degrading agents at the annual meeting of the American Society of Hematology last year and plans to advance the project into the clinical phase in the first half of 2020. This strategy can also be applied to other backbone proteins, including RIP protein kinases, and other non-enzymatic proteins and transcription factors.
The target of the central nervous system is becoming the direction of researchers. For example, drug developers are already trying to develop antibodies, antisense therapies, or gene therapy-based approaches to knock down tau and alpha-synuclein. However, these treatments have challenges in crossing the blood-brain barrier and the manner of administration. Targeted degradation agents may be a better research and development direction. Arvinas has made progress in developing compounds that target tau proteins that cross the blood-brain barrier. The company is also developing targeted degradation agents that target alpha-synuclein for the treatment of Parkinson's disease.
C4 Therapeutics and Biogen also collaborated in January to develop targeted protein degradation therapies for Alzheimer's disease and Parkinson's disease.
And this technology has broader prospects, Dr. Mainolfi said: "We have the opportunity to knock down a wide range of targets in the body with oligonucleotides and CRISPR therapy that are not currently available. And we are achieving this goal. At the same time, it also has the plasticity and scalability of small molecule compounds. This may be the most disruptive technology in the field of drug development." 

The potential and limitations of protein degradation therapy into the clinic
Prev Article
March strawberry field management points