Tetanus Shot,Tetanus Vaccine,Hepatitis B Injection,Hep B Vaccine FOSHAN PHARMA CO., LTD. , https://www.fspharmaapi.com
The essence of an antibody is immunoglobulins , which refers to a globulin having an antibody (Ab) activity or chemical structure similar to an antibody molecule. The antibody drug is a targeted drug obtained by humanizing an antibody specific for a certain disease. Immunoglobulin is generally a peptide chain structure formed by two identical light chains and two identical heavy chains linked by interchain disulfide bonds. It is divided into five categories, namely immunoglobulin G (IgG) and immunoglobulin. Protein A (IgA), immunoglobulin M (IgM), immunoglobulin D (IgD), and immunoglobulin E (IgE) . See Table 1 for details. 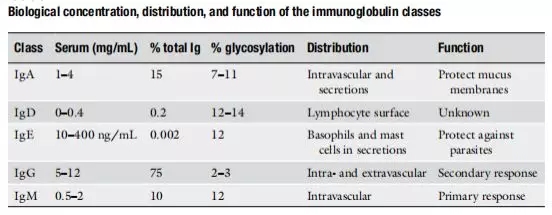
These immunoglobulins have different functions and distributions. For example, IgG is distributed in serum and tissues . It is our main type of antibody against pathogens and plays an important role in secondary defense. IgA is important for mucosal immunity. The most mysterious thing is of course IgD. Until now, the function has not been fully explained. Interested partners can study it.
There are various subtypes in various types of antibodies. For example, IgG has four subtypes in the human body. It is classified into IgG1, IgG2, IgG3, and IgG4 in our human body, and IgG1 in mice. IgG2a, IgG2b, IgG3. IgA has two subtypes, IgA1 and IgA2, as shown in Table 2. 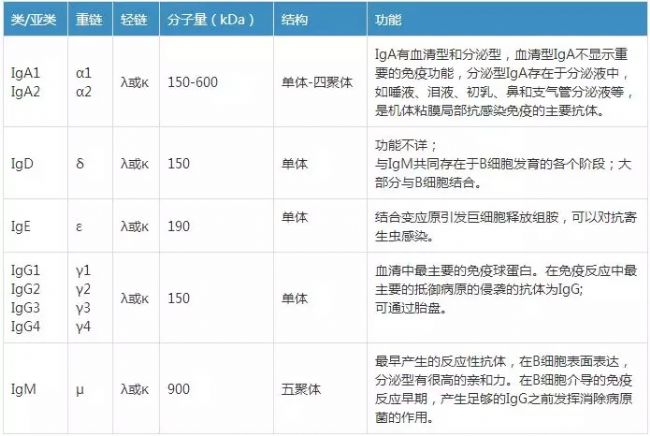
So how is the antibody subtype different? Take IgG for example. It turns out that the number and location of disulfide bonds vary among different types of IgG subtypes , as shown in Figure 1. Disulfide bonds are generally distributed in the hinge and upper CH2 domains to participate in binding to the IgG-Fc receptor (FcyR) and serum complement Clq. Antibody-dependent cell-mediated cytotoxicity (ADCC) is then initiated by FcγR binding to downstream albumin. 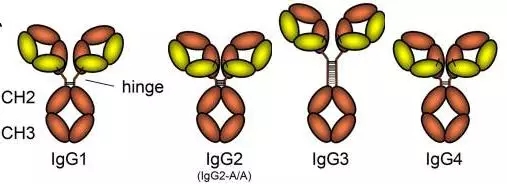
Four different structures bring different FcγR receptor binding abilities to the IgG subtype, and the stronger the receptor binding ability, the stronger the degree of immune response . In the above figure, we can see that the hinge region of IgG3 is particularly "obtrusive" compared to others, but its binding ability to FcγR receptor is the weakest, so it is often avoided in antibody drug development. This subtype of IgG3 is more biased to the subtype structure of IgG1 and IgG4 . Here to tell you, at the time, the treatment of Carter's Keytruda is actually an IgG4 modified antibody. So how should antibody drugs be selected for antibody subtypes? Please look at the officials and do not worry, let's break down next time.
In addition to the four subtypes of IgG, there are two subtypes of IgA: IgA1 and IgA2 . IgA can be present in serum or secreted as a secreted protein into the mucosa and part of body fluids. Among them, IgA1 is higher in serum and dominant in most lymphocytes; while IgA2 is higher in secretory lymphocytes , such as intestinal lymphoid tissue; heavy chain and light chain of IgA2 are not linked by disulfide bonds. Instead, link through non-covalent keys. The components of the secretions are different. In some secretions, IgA1 is more, and some IgA2 is dominant.
In serum, IgA exists in a monomeric state , as shown in Figures A and B below. In addition to the monomers, IgA may also exist as a dimer (see Figure C, D below) or even a tetramer . In the dimeric form, the C-terminus of one heavy chain of two monomers extends out of a 18-amino acid tailpiece to form a J-chain through a disulfide bond (yellow part in Figure 2C), sometimes a secretory component (short peptide). Also involved are the fixed dimeric structures (dark blue part in Figure 2D). 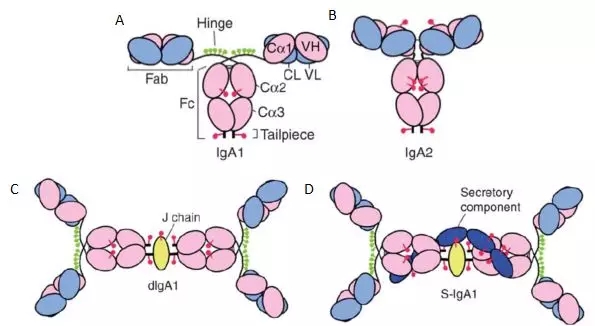
The monomer structure of IgA1 is as shown in Figure A, and the monomer structure of IgA2 is as shown in Figure B above. The pink part is the heavy chain, the light blue part is the light chain, the C picture shows the dimeric form of IgA1; the D picture shows the secretory structure of IgA1, which increases the secretory component. The red columnar structure indicates an N-terminal oligosaccharide, and the green columnar structure indicates an O-terminal oligosaccharide. The oligosaccharide plays an important role in maintaining the antibody structure, and will not be described herein.
Other immunoglobulins do not have so many siblings. There are only one subtypes like IgD, IgE, and IgM. Their structure is shown in Figure 3. 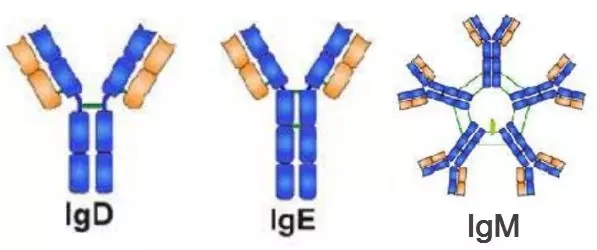
Immunoglobulin D is generally co-expressed with IgM on immature B lymphocyte membranes. IgD is similar to IgA and is a secreted immunoglobulin. Its function is to activate B cells , which may also play an important role in allergic reactions.
Immunoglobulin E (IgE) is only found in mammals. IgE is composed of two heavy chains and two light chains. The main function of IgE is immune parasites, such as Trichinella, liver fluke, etc., and IgE is hypersensitivity. It also plays an important role in the process. IgE is generally the least abundant immunoglobulin species.
Immunoglobulin M (IgM) is the largest antibody produced by vertebrates and the first antibody to appear in the initial exposure of an antigen. IgM plays an important role in the pathway of complement involvement.
Ok, today's introduction is here, the little friends interested in antibody drugs don't forget to lock the Biographs, watch the next episode - about antibody subtype classification and antibody drug screening! For more information, please visit the official website of the Olympics and Wikipedia. For the development of antibody drugs, Biotech has built a tumor antibody drug development platform and a drug pharmacology drug efficacy testing platform. Committed to better research and development of tumor antibody drugs, Biotech has been working hard!
Click here for the details of the Biotech antibody development platform
I want to know more about the "blue characters" under the dry goods.
[Dry goods] Live imaging - let the tumor cells have nowhere to shape [dry goods] mouse tumor models, those things [dry goods] to save your injured DNA -NHEJ and HR
References and image sources
[1] Robert Hnasko (ed.), ELISA: Methods and Protocols, Methods in Molecular Biology, vol. 1318,
[2] http://
[3] Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions [J]. Frontiers in immunology, 2014, 5: 520.
[4] Woof JM, Russell M W. Structure and function relationships in IgA [J]. Mucosal immunology, 2011, 4(6): 590.
[5] http://
Link: http:// 
Scan code to pay attention to the 100 Olympics map to learn more about consulting
[Dry goods] Do you know what is the protection of our antibodies every day?
Winter is coming, after a few days of cliff-type cooling, do you have a cold? In fact, everyone has a variety of antibodies in the body to protect everyone's health. And antibody drugs have sprung up, and even save the former US President Carter suffering from melanoma between critical illness. So everyone inevitably asks: What are the antibodies, what subtypes and classifications are there, what are their roles, and what is the relationship between them and antibody drugs? Today we will take a look at science for everyone.
Table 1. Function and distribution of five Igs [1]
Immunoglobulin G (IgG)
Table 2. Subtypes of various immunoglobulins [2]
Figure 1. Schematic representation of subtype structure in human IgG4 [3]
Immunoglobulin A (IgA)
Figure 2. Three-dimensional structure of human IgA1 and IgA2 [4]
Figure 3. Structure of IgD, IgE, IgM [5]
Immunoglobulin D (IgD)
Immunoglobulin E (IgE)
Immunoglobulin M (IgM)
Next Article
Drug modification also has a "butterfly effect"
Prev Article
Cultivation technology of green stem