Collapsible Foot Spa,Foot Spa Bath Massager,Foot Bath Spa Massager,Foot Soak Tub Huaian Mimir Electric Appliance Co., LTD , https://www.mimirfootbath.com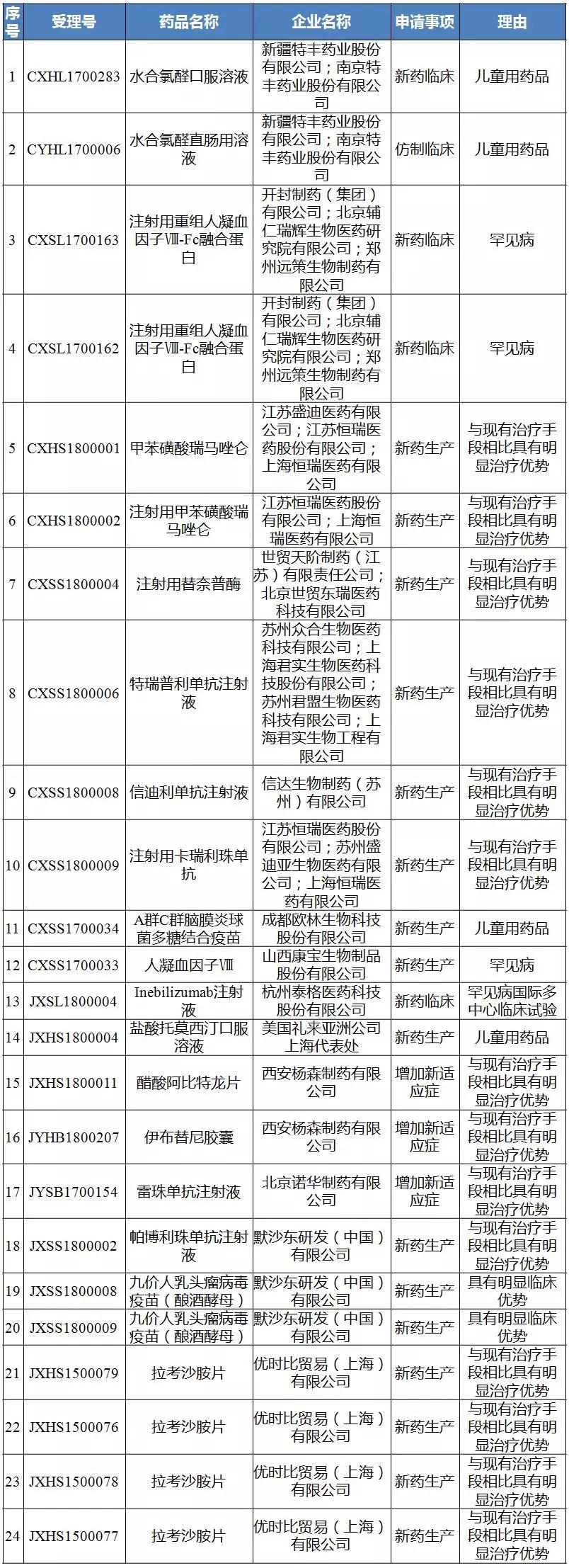
The latest proposed priority review list publicizes 3 domestic PD-1 varieties in the list!
Medical Network April 24th April 23rd, CDE official website released the twenty-eighth batch of public notices to be included in the priority review process drug registration application. Recently, several PD-1 varieties have been listed on the list, such as the Karelizumab (PD-1) for injection and the Treipril monoclonal antibody injection (PD-1). ), Cinda Bios's Dilibizumab Injection (PD-1).
Twenty-eighth batch of public notices to be included in the priority review process for drug registration applications
Source: CDE official website
It is worth noting that the Hengrui PD-1 monoclonal antibody Camrelizumab injection (SHR-1210), which is to be included in the list of the first review procedures for drug registration applications (SHR-1210), was acquired on the same day (CXSS1800009). CDE undertakes acceptance.
So far, five companies have applied for PD-1 monoclonal antibody in China, and BMS Opdivo has already taken the lead in obtaining the priority review.
New competitors after the first echelon
According to the Insight database, Hengrui's SHR-1210 (Camrelizumab) was first submitted for clinical application in 2015/1/19, and clinical approval was obtained on 2016/2/4. The indication was advanced solid tumor. In subsequent clinical trials, Hengrui registered 20 SHR-1210-related clinical studies involving single-use and combination therapy for multiple tumor indications. Among them, non-small cell lung cancer, esophageal cancer, and hepatocellular carcinoma have reached the stage III.
On April 19th, Cinda Biotech's re-submitted application for the listing of PD-1 monoclonal antibody Dilibizumab injection (CXSS1800008) was officially accepted by the Drug Evaluation Center. This is the first time that the application for the initial listing of the Sentinel has been voluntarily withdrawn in the past two months or so. The industry believes that preparation will be more adequate and the results are worth looking forward to.
On December 13, 2017, Cinda Bio's initial listing application of Idimizumab Injection (IBI308) was accepted by CDE and was the first domestic PD-1/PD-L1 mAb listing application.
Baekje Shenzhou recently announced that it is investigating the first patient in a global phase 2 clinical trial of the PD-1 antibody, tislelizumab, for patients with previously treated advanced hepatocellular carcinoma (HCC or liver cancer).
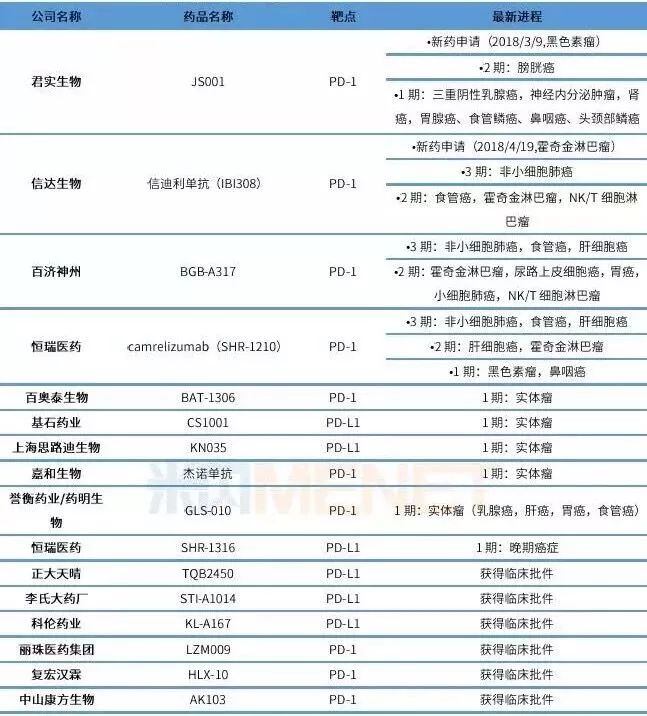
According to statistics, there are 164 new PD-1 products in the global market so far, and 1502 clinical trials involving PD-1/L1 are underway, including 1105 joint drug studies. The progress of Chinese pharmaceutical companies in the development of PD-1/L1 products is being strengthened. At present, more than 10 companies have carried out clinical trials. Among them, Junshi Bio, Cinda Bio, Hengrui Medicine and Baekje Shenzhou are in the first echelon of research and development, and a number of Chinese local companies have been added behind the four leaders. The competition is fierce. According to statistics, there are currently 25 domestic PD-1/PD-L1 monoclonal antibodies in full swing.
  Domestic enterprise PD-1/PD-L1 monoclonal antibody research and development status list
(Data source: comprehensive review of the intranet, statistics of domestic clinical trials)
Domestic varieties are expected to compete with imported varieties at the same starting line
According to PharmCube's forecast, in the first half of 2019, Cinda Bio, Junshi Bio, Baekje Shenzhou and Hengrui Medicine will all put their PD-1/L1 on the market.
PD-1 has become a superstar in the anti-cancer drug market and a shining star in cancer immunotherapy. Since its launch in 2014, Opdivo and Keytruda have only three full years, with total sales in 2017 reaching $8.757 billion. According to Evaluate Pharma, Opdivo and Keytruda are expected to have a combined revenue of more than $19.4 billion in 2022 and a compound growth rate of 17% in 2017-2022, making it the market's most potential anti-tumor targeted drug.
As the pioneer of tumor immunotherapy, PD-1/PD-L1 monoclonal antibody has been highly sought after by the market and has become a must for pharmaceutical companies. Who will become the first domestic PD-1/PD-L1 monoclonal antibody approved for listing?
According to analysis in the industry, according to the clinical progress and the time of submitting the application for listing, domestic anti-PD-1 monoclonal antibody and imported varieties including Junshi Bio, Hengrui Medicine, Baekje Shenzhou and Cinda Bio are only 4-6 months apart, and the future The approved time to market will also be small. Domestic varieties are expected to compete with imported varieties at the same starting line, and occupy the main market with the advantage of cost performance.