The second female "Viagra" will be listed in the US, but does it really work? June 28, 2019 Source: Research Circle Last week, the US Food and Drug Administration (FDA) approved a drug, bromelanotide (Vyleesi), which enhances female sexual desire. This is the second drug approved for the treatment of hypoactive sexual desire disorder (HSDD) in the United States, and the pricing is not yet public. "Sexually cold" saver? According to Sharon Parish, a professor of clinical psychiatry at Weill Cornell Medical Center, about 6% to 10% of women of childbearing age worldwide may have HSDD. It is one of the most common types of female sexual dysfunction (FSD), which is mainly characterized by decreased sexual desire and difficulties in emotional anxiety and interpersonal relationships. From a biological point of view, the generation of sexual desire and the excitability of the brain are closely related to the integrated regulation of inhibitory neurons. In women who are plagued by HSDD, there is a “suppression system disorder†in the nervous system. Vyleesi activates melanocortin 4, which regulates sexual desire and sexual response through endogenous pathways in the brain, helping premenopausal women maintain normal sexual desire. This sounds great, but according to Bloomberg News, the FDA disclosed Vyleesi clinical trial data is a bit thin – more than 1,200 participants, 25% of patients in the medication group think their libido is improved, in placebo The group number is 17%. This has led some public to question the significance of the existence of this drug. Leonore Tiefer, a sexual therapist from New York, believes that the loss of libido is not a purely biological problem. It is not easy to solve the problem by “stepping on the gas pedalâ€. But the drug manufacturer, AMAG Pharmaceuticals, says they haven't planned to make a huge improvement from the start, because even seemingly minor changes can have a major impact on the mental state and quality of life of women with HSDD. Forecourt Vyleesi is not the first female "Viagra" to enter the doctor's prescription and pharmacy medicine rack. In 2015, the US FDA approved the world's first oral drug, Flibanserin (brand name Addyi), to treat HSDD and improve women's quality of life. Addyi's target is different from Vyleesi, which works by inhibiting serotonin (5-HT) activity in the brain. There have been studies (a review published in The Journal of Sexual Medicine in 2017) that 5-HT can reduce sexual motivation by inhibiting the excitatory dopaminergic system. Therefore, regulation of 5-HT and its receptors in the brain can theoretically improve "sexual coldness." Although the listing of the first female version of Viagra attracted a lot of attention, the performance of Addyi after the listing was disappointing: there was a voice accusing the FDA of not being strict in the approval process, and the drug was slower to produce, and it also produced alcohol. The unclear side effects make it lose a lot of potential users. In 2018, the drug's global sales of $3.48 million (IQVIA MIDASTM data) - less than one-sixth of Pfizer's famous "Blue Pill" 2018 sales (Sidinafil, trade name Ai Wanke, legend "Viagra" in the 2018 sales of $ 217 million). Vyleesi does not have Addyi's alcohol safety problem, but the former is still a barrier to acceptance. It is an injectable drug, and patients must give themselves a subcutaneous injection before sexual activity to be effective. In addition, the most common side effect of the drug is nausea, which is shown in approximately 40% of women in clinical trials. The FDA recommends that women use only one dose within 24 hours, and the monthly dose should not exceed 8 doses. Even if the inconvenient experience and the poor performance of the “predecessor†Addyi can be ignored, Vyleesi's popularization path still has an important problem to be solved: as a drug for the treatment of female HSDD, it may not find enough HSDD patients. . At present, this female sexual dysfunction disease is diagnosed by exclusion, which is a very complicated process. First, patients must be aware of their loss of libido and feel painful. Second, doctors need to rule out other factors that lead to libido, such as the breakdown of intimate relationships, the side effects of other drugs, or the persistent effects of surgery. . A business analyst told STAT that although about 5 million women in the United States may become potential consumers of this new drug, it is difficult to know what percentage of women actually seek treatment, which may be a very small number. Entering China? Although Wall Street analysts are not very optimistic about Vyleesi's business prospects, there are still companies interested in this injection-type female "Viagra". In 2017, Shanghai Fosun Pharmaceutical acquired the exclusive commercialization and non-exclusive development and manufacturing rights of bumene in China from Palatin Technologies for US$105 million (Vyleesi's manufacturer AMAG Pharmaceuticals also from Palatin in the same year) Technologies obtained the commercialization of the drug in the United States). At present, China has not approved any drugs for the treatment of HSDD, and some medical media believe that there is no “blue ocean†in this field in China. In April 2019, Fosun Pharma announced that its application for “Bumenotide Injection for the Treatment of Women's Sexual Disorders†has been accepted by the State Drug Administration for clinical trial registration. The future of the dispute Cancer therapy can shrink tumors, diabetes drugs can lower blood sugar, then Vyleesi's "sexually modified drugs" will certainly "save" the decline of desire? (Extended reading: BMJ: Modern human life is getting less and less, especially not single) Dr. Sharon Parish, who worked for the AMAG as a paid consultant at the New York-Presbyterian Hospital, believes that for women with HSDD, there is no such thing as creating a romantic atmosphere and having a partner with a glass of wine. Any help, this is a truth that it is impossible for a depressed patient to be cured by watching a funny movie. They should get help from drugs to get rid of the pain and distress caused by HSDD. However, we must admit that the desire for sex is not just a matter of the physical level. According to Leonore Tiefer, a sexual therapist from New York, it is a mixture of complex factors such as the reproductive organs, the circulatory system, the nervous system, intimacy, humanity, marital status, etc. It is even hard to define what is “sexâ€. This is a bit like dancing - no one will use anatomical methods to explain why ballet can bring us pleasure, which is ridiculous. Sheryl Kingsberg, a clinical psychologist at the Cleveland Medical Center in the United States, is much more optimistic: the emergence of women such as sexual desire-improving drugs such as Vyleesi and Addyi allows people to face up to the possible existence of women in their sexual lives. The pain of being difficult to talk about. Just as in the history of modern medicine, the drug "Prozac", which treats depression, has experienced twists and turns and become an important change in the psychiatric world. She hopes that the female "Viagra" can also bring the diagnosis and treatment standards for future female sexual behavior disorder. change. Growth Promtor,Growth Promoter For Poultry,Growth Promoter Hormone,Growth Promoter For Cattle Sichuan Aibang Weiye Biological Engineering Co., Ltd. , https://www.aibangpharm.com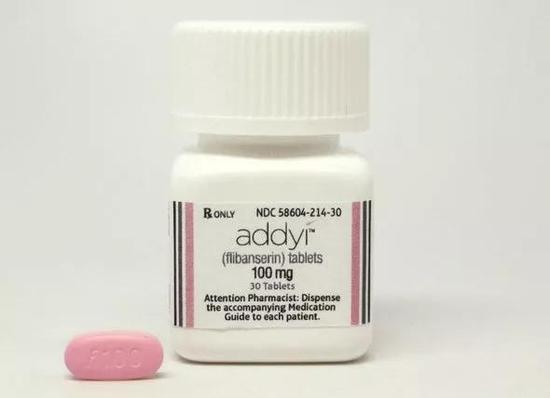
Image source: ScientificAmerican
The second female "Viagra" will be listed in the US, but does it really work?
Next Article
Northeastern greenhouse melon cultivation technology
Prev Article
Remove the side notes of the tomato